High sensitivity mapping of brain-wide functional networks in awake mice using simultaneous multi-slice fUS imaging
- PMID: 40799722
- PMCID: PMC12007538
- DOI: 10.1162/imag_a_00030
High sensitivity mapping of brain-wide functional networks in awake mice using simultaneous multi-slice fUS imaging
Abstract
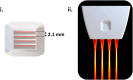
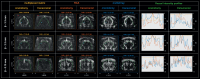
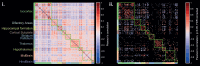
Functional ultrasound (fUS) has received growing attention in preclinical research in the past decade, providing a new tool to measure functional connectivity (FC) and brain task-evoked responses with single-trial detection capability in both anesthetized and awake conditions. Most fUS studies rely on 2D linear arrays to acquire one slice of the brain. Volumetric fUS using 2D matrix or row-column arrays has recently been demonstrated in rats and mice but requires invasive craniotomy to expose the brain due to a lack of sensitivity. In a previous study, we proposed the use of motorized linear arrays, allowing imaging through the skull in mice for multiple slices with high sensitivity. However, the tradeoff between the field of view and temporal resolution introduced by motorized scanning prevents acquiring brain-wide resting-state FC data with a sufficient volume rate for resting-state FC analysis. Here, we propose a new hybrid solution optimized and dedicated to brain-wide transcranial FC studies in mice, based on a newly developed multi-array transducer allowing simultaneous multi-slicing of the entire mouse cerebrum. We first demonstrate that our approach provides a better imaging quality compared to other existing methods. Then, we show the ability to image the whole mouse brain non-invasively through the intact skin and skull during visual stimulation under light anesthesia to validate this new approach. Significant activation was detected along the whole visual pathway, at both single and group levels, with more than 10% of augmentation of the cerebral blood volume (CBV) signal during the visual stimulation compared to baseline. Finally, we assessed resting-state FC in awake head-fixed animals. Several robust and long-ranged FC patterns were identified in both cortical and sub-cortical brain areas, corresponding to functional networks already described in previous fMRI studies. Together, these results show that the multi-array probe is a valuable approach to measure brain-wide hemodynamic activity in mice with an intact skull. Most importantly, its ability to identify robust resting-state networks is paving the way towards a better understanding of the mouse brain functional organization and its breakdown in genetic models of neuropsychiatric diseases.
Keywords: awake mice; brain imaging; connectomics; functional connectivity; functional ultrasound; visual pathway; volumetric imaging.
© 2023 Massachusetts Institute of Technology.
Conflict of interest statement
Mickael Tanter, Mathieu Pernot, Bruno-Félix Osmanski, and Thomas Deffieux are share-holders and co-founders of the Iconeus company. Adrien Bertolo, Jeremy Ferrier, Samuel Diebolt, and Bruno-Félix Osmanski are employees of the Iconeus company.
Figures






Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
Cited by
-
Functional ultrasound (fUS) detects mild cerebral alterations using canonical correlation analysis denoising and dynamic functional connectivity analysis.Imaging Neurosci (Camb). 2025 Sep 2;3:IMAG.a.128. doi: 10.1162/IMAG.a.128. eCollection 2025. Imaging Neurosci (Camb). 2025. PMID: 40909354 Free PMC article.
References
-
- Aydin, A.-K., Haselden, W. D., Goulam Houssen, Y., Pouzat, C., Rungta, R. L., Demené, C., Tanter, M., Drew, P. J., Charpak, S., & Boido, D. (2020). Transfer functions linking neural calcium to single voxel functional ultrasound signal. Nature Communications, 11(1), 2954. 10.1038/s41467-020-16774-9 - DOI - PMC - PubMed
-
- Bertolo, A., Nouhoum, M., Cazzanelli, S., Ferrier, J., Mariani, J.-C., Kliewer, A., Belliard, B., Osmanski, B.-F., Deffieux, T., Pezet, S., Lenkei, Z., & Tanter, M. (2021). Whole-brain 3D activation and functional connectivity mapping in mice using transcranial functional ultrasound imaging. Journal of Visualized Experiments, 168, 62267. 10.3791/62267 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
