Stool-Based Molecular Tuberculosis Treatment Monitoring: A Faster Means for Detecting Persistent Mycobacteria Compared to Phenotypic Culture
- PMID: 40799783
- PMCID: PMC12342930
- DOI: 10.1093/ofid/ofaf345
Stool-Based Molecular Tuberculosis Treatment Monitoring: A Faster Means for Detecting Persistent Mycobacteria Compared to Phenotypic Culture
Abstract
Background: Tuberculosis (TB) treatment monitoring is hindered by the lack of a rapidly measured biomarker that accurately predicts clinically relevant outcomes. Symptom screening poorly correlates with bacillary burden. Although culture is a direct measure of viable bacillary burden, the long turnaround time makes it clinically irrelevant.
Methods: The TB treatment monitoring potential of stool-based, quantitative polymerase chain reaction (qPCR) was prospectively assessed among 231 participants of all ages from Eswatini, Tanzania, and Mozambique with microbiologically confirmed TB. Stool qPCR results were compared to sputum culture, persistent symptoms, drug resistance, and World Health Organization TB outcomes.
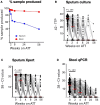
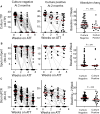
Results: Quantitative bacillary burden measured by stool qPCR strongly correlated with sputum culture at baseline (Spearman correlation r s = 0.79; P < .001). Stool was successfully collected at >90% of all timepoints, while sputum collection decreased to <50% at the end of therapy. Participants with isoniazid or rifampin resistance demonstrated decreased bacillary clearance by sputum culture and stool qPCR during the first 2 weeks of treatment. Participants who remained culture positive at 2 months had a slower decrease in bacillary burden measured by stool qPCR compared to those who were culture negative by 2 months. The odds of a participant being culture positive at 2 months was associated with a lower initial qPCR cycle threshold (odds ratio [OR], 0.792; P = .004), and a smaller absolute difference between the qPCR cycle threshold measured at 2 weeks and baseline (OR, 0.72; P = .0006). Neither sputum culture, sputum Xpert Ultra, or stool qPCR was associated with resolution of symptoms or in-treatment death.
Conclusions: Stool-based TB treatment monitoring correlates with sputum culture but provides results faster, leverages a more accessible specimen, and identifies patients with TB who are at risk for drug resistance and persistent 2-month culture positivity. None of the quantitative tests of bacillary burden singularly could predict symptom resolution or death.
Keywords: sputum culture; stool qPCR test; stool-based; stool-based molecular test; treatment monitoring.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures


References
-
- MacLean EL, Zimmer AJ, den Boon S, et al. Tuberculosis treatment monitoring tests during routine practice: study design guidance. Clin Microbiol Infect 2024; 30:481–8. - PubMed
-
- Gillespie SH, DiNardo AR, Georghiou SB, et al. Developing biomarker assays to accelerate tuberculosis drug development: defining target product profiles. Lancet Microbe 2024; 5:100869. - PubMed
LinkOut - more resources
Full Text Sources

