Medical Hemorrhoid Gel Ameliorates Croton Oil-Induced Hemorrhoids by Suppressing the NLRP3 Inflammasome Activation via NF-κB Signaling Pathway
- PMID: 40799797
- PMCID: PMC12339917
- DOI: 10.2147/JIR.S526589
Medical Hemorrhoid Gel Ameliorates Croton Oil-Induced Hemorrhoids by Suppressing the NLRP3 Inflammasome Activation via NF-κB Signaling Pathway
Abstract
Background: Hemorrhoidal disease (HD) is characterized by the pathological dilation of anal vascular cushions, causing pain, itching and bleeding. Recent evidence links HD onset and progression to rectal inflammation. Medical Hemorrhoid Gel (MHG), a multi-component botanical preparation, has gained empirical validation for HD management. This study aims to systematically evaluate the safety, efficacy, and mechanism of action of MHG in treating HD.
Methods: The phytochemical composition of MHG was characterized using UPLC-QTOF-MS/MS and GC-MS/MS analyses. In vivo, the efficacy was assessed in a croton oil preparation (COP)‑induced HD rat model (n=10 per group) via anorectal coefficient (ARC) measurement, macroscopic severity score, Evans blue extravasation quantification, and H&E/PAS staining. Transcriptomic sequencing of anorectal tissues was integrated with experimental validation using ELISA, immunohistochemistry (IHC), and Western blotting to delineate molecular mechanism. Data were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison post hoc test (significance at p<0.05).
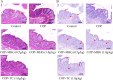
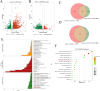
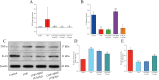
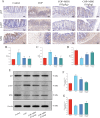
Results: MHG significantly reduced ARC, macroscopic severity score, and Evans blue extravasation, restored intestinal villus structure and goblet cell numbers, and alleviated inflammation. Acute toxicity tests showed that MHG did not cause anorectal abnormalities or systemic toxicity in rats. Transcriptomic analysis integrated with experimental validation suggested the therapeutic mechanism of MHG involves inflammation response and NF-κB pathway. Specifically, MHG suppressed the levels of the pro-inflammatory mediator TNF-α, while it enhanced the levels of the anti-inflammatory mediator IL-10. Mechanistic studies revealed that MHG inhibited NLRP3 inflammasome activation, reduced the phosphorylation level of p65 and enhanced IκBα expression. Phytochemical analysis identified 20 constituents that contribute to the bioactivity of MHG.
Conclusion: Our study substantiated that MHG exerts anti-hemorrhoidal effects through NLRP3 inflammasome suppression via NF-κB pathway regulation. This mechanistic insight provides scientific validation for clinical application of MHG in HD management.
Keywords: NK-κB signaling pathway; NLRP3 inflammasomes; hemorrhoidal disease; inflammation; medical hemorrhoid gel.
© 2025 Ai et al.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures




Similar articles
-
Mahonia bealei (Fort.) Carr. Leaf extract modulates the TLR2/MyD88/NF-κB signaling pathway to inhibit PGN-induced inflammation in RAW264.7 cells.J Ethnopharmacol. 2025 Mar 26;344:119510. doi: 10.1016/j.jep.2025.119510. Epub 2025 Feb 17. J Ethnopharmacol. 2025. PMID: 39971016
-
Sub-chronic realgar exposure causes liver inflammatory injury in mice by inducing bile acid-mediated NLRP3 inflammasome activation through down-regulation of ileal FXR.J Ethnopharmacol. 2025 Jul 24;351:120174. doi: 10.1016/j.jep.2025.120174. Epub 2025 Jun 18. J Ethnopharmacol. 2025. PMID: 40541751
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Recurrence Rates and Pharmacological Treatment for Hemorrhoidal Disease: A Systematic Review.Adv Ther. 2023 Jan;40(1):117-132. doi: 10.1007/s12325-022-02351-7. Epub 2022 Nov 4. Adv Ther. 2023. PMID: 36331754 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

