Sustained release of cathepsin D/cathepsin K from injectable hydrogel microspheres in anti-photoaging
- PMID: 40799995
- PMCID: PMC12341577
- DOI: 10.1016/j.mtbio.2025.102150
Sustained release of cathepsin D/cathepsin K from injectable hydrogel microspheres in anti-photoaging
Abstract
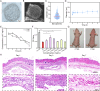
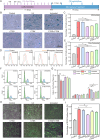
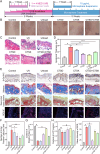
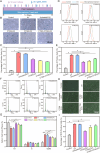
There have been various interventions for photoaging skin, such as retinoic acid treatment and laser therapy. However, more precise, secure, and effective technologies and materials were still need to be explored. Cathepsin D (CTSD) repairs the epidermal barrier in chronic photodamaged skin through increasing TGase-1 expression and activity. Cathepsin K (CTSK) affects the metabolism of skin elastic fibers. Therefore, gelatin/alginate composite hydrogel microspheres loaded with CTSD or CTSK were fabricated. The microspheres with an average size of 79.8 ± 30.4 μm were stable in PBS (pH 7.4) for 5 days and then disintegrated in vitro. 0.1 mg/mL of microspheres continuously released 12-13 ng/mL CTSD or CTSK for the first three consecutive days. In vivo, the microspheres were almost completed degraded on day 7 after the subcutaneous injection. The biosafety and efficacy of CTSD- and CTSK- microspheres were confirmed in vitro and in vivo. They reduced ROS and inhibited the degradation of skin collagen and elastic fibers by upregulating Nrf2 and downregulating MMP-1 and MMP-3, which reversed photodamage of skin induced by UV. In conclusion, the gelatin/alginate composite microspheres loaded with CTSD or CTSK showed a great potential for chronic photodamaged skin.
Keywords: Cathepsin D; Cathepsin K; Degradable microspheres; Photoaging; Skin.
© 2025 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Yue Zheng reports financial support was provided by National Natural Science Foundation of China. Yue Zheng reports financial support was provided by Science and Technology Program of Guangzhou. Yue Zheng reports financial support was provided by 10.13039/501100003453Natural Science Foundation of Guangdong Province. Yue Zheng has patent Enzyme-Loaded Controlled-Release Microspheres for Skin Photoaging Prevention/Treatment: Preparation Method and Applications pending to Yue Zheng. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Shen Y.J., Kim K., Zhu Z.X., Zhang S.Y., Jiang M.Y., Liu Z.L., Zheng Y.Y., Li X.K., Jin L.T., Cong W.T. δ-Catenin requirement in keratinocyte proliferation and DNA repair identifies a therapeutic target for photoaging. J. Invest. Dermatol. 2023;143(1):26. doi: 10.1016/j.jid.2022.07.009. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

