CDCP1-targeting ADC outperforms standard therapies in Ras-mutant pancreatic cancer
- PMID: 40800005
- PMCID: PMC12341598
- DOI: 10.1016/j.omton.2025.201024
CDCP1-targeting ADC outperforms standard therapies in Ras-mutant pancreatic cancer
Abstract
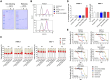
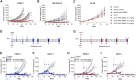
R AS mutations are found in 10%-30% of various cancers and in up to 90% of pancreatic cancers, where they are associated with aggressive phenotypes, poor prognosis, and reduced overall survival. CUB domain containing protein 1 (CDCP1), a transcriptional target of activated RAS, is implicated in these cancers irrespective of the specific R AS mutation. Given the limited effectiveness of small-molecule inhibitors against mutant Ras-driven cancers, we developed a CDCP1-targeting antibody-drug conjugate (ADC). In this study, we demonstrate that CDCP1 overexpression significantly correlates with R AS mutations in pancreatic cancer. We generated and characterized a CDCP1-specific monoclonal antibody, 2G10, and conjugated it to the topoisomerase II inhibitor, PNU159682, to produce 2G10-PNU159682. The anti-tumor activity of this ADC was evaluated in vitro and in vivo using pancreatic cancer cell lines. 2G10-PNU159682 exhibited superior efficacy compared to MRTX1133 and sotorasib in G12D- and G12C-mutant cell lines. In a mouse xenograft model, 2G10-PNU159682 demonstrated robust anti-tumor activity against R AS-mutant pancreatic cancers, outperforming gemcitabine and FOLFIRINOX and achieving complete tumor remission for up to 100 days-even following relapse after standard chemotherapy. These findings support the potential of 2G10-PNU159682 as a promising therapeutic candidate for the treatment of Ras-mutant cancers.
Keywords: 2G10-PNU159682; CDCP1; MT: Regular Issue; Ras mutation; antibody-drug conjugate; pancreatic cancer.
© 2025 The Author(s).
Conflict of interest statement
S.G.P. is a founder and CEO of the Novelty Nobility Inc. J.G.C., H.-J.K., J.-O.K., and T.M.W. are employees of the Novelty Nobility.
Figures





References
-
- Guerrero S.l., Casanova I., Farré L., Mazo A., Capellà G., Mangues R. K-ras codon 12 mutation induces higher level of resistance to apoptosis and predisposition to anchorage-independent growth than codon 13 mutation or proto-oncogene overexpression. Cancer Res. 2000;60:6750–6756. - PubMed
-
- Khan A.Q., Kuttikrishnan S., Siveen K.S., Prabhu K.S., Shanmugakonar M., Al-Naemi H.A., Haris M., Dermime S., Uddin S. Elsevier; 2019. RAS-Mediated Oncogenic Signaling Pathways in Human Malignancies. Paper Presented at: Seminars in Cancer Biology. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
