Detection of respiration-induced field modulations in fMRI: A concurrent and navigator-free approach
- PMID: 40800327
- PMCID: PMC12224404
- DOI: 10.1162/imag_a_00091
Detection of respiration-induced field modulations in fMRI: A concurrent and navigator-free approach
Abstract
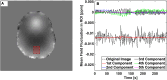
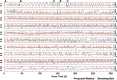
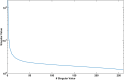
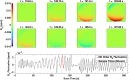
Functional Magnetic Resonance Imaging (fMRI) is typically acquired using gradient-echo sequences with a long echo time at high temporal resolution. Gradient-echo sequences inherently encode information about the magnetic field in the often discarded image phase. We demonstrate a method for processing the phase of reconstructed fMRI data to isolate temporal fluctuations in the harmonic fields associated with respiration by solving a blind source separation problem. The fMRI-derived field fluctuations are shown to be in strong agreement with breathing belt data acquired during the same scan. This work presents a concurrent, hardware-free measurement of respiration-induced field fluctuations, providing a respiratory regressor for fMRI analysis which is independent of local contrast changes, and with potential applications in image reconstruction and fMRI analysis.
Keywords: fMRI; field mapping; off-resonance; physiological noise; quantitative susceptibility mapping; respiratory monitoring.
© 2024 Massachusetts Institute of Technology. Published under a Creative Commons Attribution 4.0 International (CC BY 4.0) license.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Respiration recording for fMRI: breathing belt versus spine coil sensor.Imaging Neurosci (Camb). 2024 Jul 24;2:imag-2-00239. doi: 10.1162/imag_a_00239. eCollection 2024. Imaging Neurosci (Camb). 2024. PMID: 40800279 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Magnetic resonance perfusion for differentiating low-grade from high-grade gliomas at first presentation.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD011551. doi: 10.1002/14651858.CD011551.pub2. Cochrane Database Syst Rev. 2018. PMID: 29357120 Free PMC article.
-
MRI software and cognitive fusion biopsies in people with suspected prostate cancer: a systematic review, network meta-analysis and cost-effectiveness analysis.Health Technol Assess. 2024 Oct;28(61):1-310. doi: 10.3310/PLFG4210. Health Technol Assess. 2024. PMID: 39367754 Free PMC article.
References
-
- Ayaz, M., Boikov, A., McAuley, G., Schrag, M., Kido, D. K., Haacke, E. M., & Kirsch, W. (2011). Imaging cerebral microbleeds with SWI. In Susceptibility weighted imaging in MRI (pp. 191–214). John Wiley & Sons, Ltd. 10.1002/9780470905203.ch12 - DOI
-
- Bancelin, D., Bachrata, B., Bollmann, S., de Lima Cardoso, P., Szomolanyi, P., Trattnig, S., & Robinson, S. D. (2023). Unsupervised physiological noise correction of functional magnetic resonance imaging data using phase and magnitude information (PREPAIR). Human Brain Mapping, 44(3), 1209–1226. 10.1002/hbm.26152 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
