Identification of rare cortical folding patterns using unsupervised deep learning
- PMID: 40800369
- PMCID: PMC12224473
- DOI: 10.1162/imag_a_00084
Identification of rare cortical folding patterns using unsupervised deep learning
Abstract
Like fingerprints, cortical folding patterns are unique to each brain even though they follow a general species-specific organization. Some folding patterns have been linked with neurodevelopmental disorders. However, due to the high inter-individual variability, the identification of rare folding patterns that could become biomarkers remains a very complex task. This paper proposes a novel unsupervised deep learning approach to identify rare folding patterns and assess the degree of deviations that can be detected. To this end, we preprocess the brain MR images to focus the learning on the folding morphology and train a beta variational auto-encoder ( ) on the inter-individual variability of the folding to identify outliers. We compare the detection power of the latent space and of the reconstruction errors, using synthetic benchmarks and one actual rare configuration related to the central sulcus. Finally, we assess the generalization of our method on a developmental anomaly located in another region and we validate the relevance of our approach on patients suffering from drug-resistant epilepsy. Our results suggest that this method enables encoding relevant folding characteristics that can be enlightened and better interpreted based on the generative power of the . The latent space and the reconstruction errors bring complementary information and enable the identification of rare patterns of different nature. This method generalizes well to a different region on another dataset and demonstrates promising results on the epileptic patients. Code is available at https://github.com/neurospin-projects/2022_lguillon_rare_folding_detection.
Keywords: Folding patterns; anomaly detection; cortical folding; cortical sulci; epilepsy; unsupervised learning; β − VAE.
© 2024 Massachusetts Institute of Technology. Published under a Creative Commons Attribution 4.0 International (CC BY 4.0) license.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
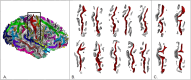

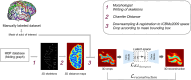




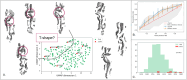

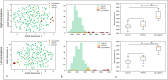




References
-
- Baur, C., Denner, S., Wiestler, B., Albarqouni, S., & Navab, N. (2020). Autoencoders for unsupervised anomaly segmentation in brain MR images: A comparative study. arXiv:2004.03271 [cs, eess] http://arxiv.org/abs/2004.03271 - PubMed
-
- Behrendt, F., Bengs, M., Rogge, F., Krüger, J., Opfer, R., & Schlaefer, A. (2022). Unsupervised anomaly detection in 3D brain MRI using deep learning with impured training data. In 2022 IEEE 19th International Symposium on Biomedical Imaging (ISBI) (pp. 1–4). 10.1109/isbi52829.2022.9761443 - DOI
-
- Bénézit, A., Hertz-Pannier, L., Dehaene-Lambertz, G., Monzalvo, K., Germanaud, D., Duclap, D., Guevara, P., Mangin, J. F., Poupon, C., Moutard, M.L., & Dubois, J. (2015). Organising white matter in a brain without corpus callosum fibres. Cortex, 63, 155–171. 10.1016/j.cortex.2014.08.022 - DOI - PubMed
LinkOut - more resources
Full Text Sources
