Awake rodent fMRI: Gradient-echo echo planar imaging versus compressed-sensing fast low-angle shot
- PMID: 40800768
- PMCID: PMC12330865
- DOI: 10.1162/imag_a_00406
Awake rodent fMRI: Gradient-echo echo planar imaging versus compressed-sensing fast low-angle shot
Abstract
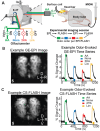
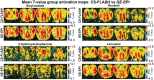
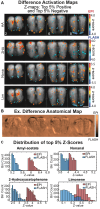
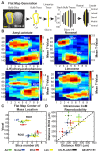
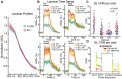
Awake rodent functional magnetic resonance imaging (fMRI) is increasingly becoming a reliable neuroimaging technique to study neuronal activity at both the whole-brain and high-resolution laminar scales. Prior studies have focused on developing acclimation protocols, experimental paradigms, and hardware to optimize outcomes. However, little effort has been made to address the impact of pulse sequence selection on detecting brain activation in awake fMRI experiments. In the current study, we compare gradient-echo echo planar imaging (GE-EPI) and compressed-sensing fast low-angle shot (CS-FLASH) sequences with cerebral blood volume-weighted (CBVw) contrast enhancement to investigate their sensitivity to hemodynamic activity in the olfactory bulb of awake rodents. Compared with GE-EPI, CS-FLASH had comparable motion parameters but was more sensitive to large motions, often resulting in corruption of the image quality. The use of framewise displacement as a motion censoring technique may over censor the data, requiring alternative approaches, such as spatial correlation censoring. CS-FLASH images were qualitatively sharper than GE-EPI; however, the contrast-to-noise ratio for odor activation was consistently greater for GE-EPI than for CS-FLASH that cannot be explained by olfactory adaptation alone. The activation maps of CS-FLASH to four different odors showed spatially unique patterns consistent with GE-EPI, but with lower z-scores or detection sensitivity. Activation maps were consistent with previously established histological findings. Additionally, odor-evoked laminar activation was greatest in the superficial layers that decreased with laminar depth, consistent with prior findings. We conclude that CS-FLASH produces sharper images with equivalent spatial activation maps to GE-EPI, albeit with lower statistical strength and contrast-to-noise ratio (CNR), and without being prohibited by motion-related image distortion.
Keywords: CBVw fMRI; awake rodent fMRI; functional pulse sequence comparison; high-resolution fMRI; olfactory bulb.
© 2024 The Authors. Published under a Creative Commons Attribution 4.0 International (CC BY 4.0) license.
Conflict of interest statement
The authors declare that they have no competing or conflicts of interest.
Figures

Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
MarkVCID cerebral small vessel consortium: II. Neuroimaging protocols.Alzheimers Dement. 2021 Apr;17(4):716-725. doi: 10.1002/alz.12216. Epub 2021 Jan 21. Alzheimers Dement. 2021. PMID: 33480157 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Three-Dimensional Single-Shot CEST Imaging at 3T Based on True FISP Readout.NMR Biomed. 2025 Sep;38(9):e70109. doi: 10.1002/nbm.70109. NMR Biomed. 2025. PMID: 40715993
References
LinkOut - more resources
Full Text Sources
