Streamline tractography of the fetal brain in utero with machine learning
- PMID: 40800807
- PMCID: PMC12319953
- DOI: 10.1162/imag_a_00537
Streamline tractography of the fetal brain in utero with machine learning
Abstract
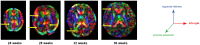
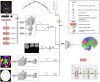
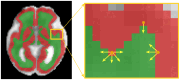
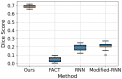
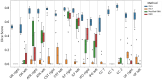
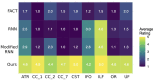
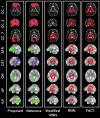
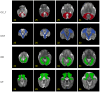
Diffusion-weighted magnetic resonance imaging (dMRI) is the only non-invasive tool for studying white matter tracts and structural connectivity of the brain. These assessments rely heavily on tractography techniques, which reconstruct virtual streamlines representing white matter fibers. Much effort has been devoted to improving tractography methodology for adult brains, while tractography of the fetal brain has been largely neglected. Fetal tractography faces unique difficulties due to low dMRI signal quality, immature and rapidly developing brain structures, and paucity of reference data. To address these challenges, this work presents a machine learning model, based on a deep neural network, for fetal tractography. The model input consists of five different sources of information: (1) Voxel-wise fiber orientation, inferred from a diffusion tensor fit to the dMRI signal; (2) Directions of recent propagation steps; (3) Global spatial information, encoded as normalized distances to keypoints in the brain cortex; (4) Tissue segmentation information; and (5) Prior information about the expected local fiber orientations supplied with an atlas. In order to mitigate the local tensor estimation error, a large spatial context around the current point in the diffusion tensor image is encoded using convolutional and attention neural network modules. Moreover, the diffusion tensor information at a hypothetical next point is included in the model input. Filtering rules based on anatomically constrained tractography are applied to prune implausible streamlines. We trained the model on manually-refined whole-brain fetal tractograms and validated the trained model on an independent set of 11 test subjects with gestational ages between 23 and 36 weeks. Results show that our proposed method achieves superior performance across all evaluated tracts. Qualitative assessments on independent data from the Developing Human Connectome Project demonstrated the generalizability of our method. The new method can significantly advance the capabilities of dMRI for studying normal and abnormal brain development in utero.
Keywords: developing brain; diffusion MRI; fetal brain; machine learning; tractography.
© 2025 The Authors. Published under a Creative Commons Attribution 4.0 International (CC BY 4.0) license.
Conflict of interest statement
The author has no conflicts of interest to declare.
Figures








Update of
-
Streamline tractography of the fetal brain in utero with machine learning.ArXiv [Preprint]. 2024 Aug 26:arXiv:2408.14326v1. ArXiv. 2024. Update in: Imaging Neurosci (Camb). 2025 Apr 09;3:imag_a_00537. doi: 10.1162/imag_a_00537. PMID: 39253631 Free PMC article. Updated. Preprint.
Similar articles
-
Streamline tractography of the fetal brain in utero with machine learning.ArXiv [Preprint]. 2024 Aug 26:arXiv:2408.14326v1. ArXiv. 2024. Update in: Imaging Neurosci (Camb). 2025 Apr 09;3:imag_a_00537. doi: 10.1162/imag_a_00537. PMID: 39253631 Free PMC article. Updated. Preprint.
-
Tractography from T1-weighted MRI: Empirically exploring the clinical viability of streamline propagation without diffusion MRI.Imaging Neurosci (Camb). 2024 Aug 13;2:imag-2-00259. doi: 10.1162/imag_a_00259. eCollection 2024. Imaging Neurosci (Camb). 2024. PMID: 40800467 Free PMC article.
-
Mapping Caudolenticular Gray Matter Bridges in the Human Brain Striatum Through Diffusion Magnetic Resonance Imaging and Tractography.Hum Brain Mapp. 2025 Jun 1;46(8):e70245. doi: 10.1002/hbm.70245. Hum Brain Mapp. 2025. PMID: 40459517 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Avants , B. B. , Epstein , C. L. , Grossman , M. , & Gee , J. C. ( 2008. ). Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain . Medical Image Analysis , 12 ( 1 ), 26 – 41 . 10.1016/j.media.2007.06.004 - DOI - PMC - PubMed
-
- Bodini , B. , & Ciccarelli , O. ( 2009. ). Diffusion MRI in neurological disorders . In Johansen-Berg H. & Behrens T. E. J. (Eds.), Diffusion MRI (pp. 175 – 203 ). Elsevier. 10.1016/b978-0-12-374709-9.00009-2 - DOI
-
- Cai , L. Y. , Lee , H. H. , Newlin , N. R. , Kerley , C. I. , Kanakaraj , P. , Yang , Q. , Johnson , G. W. , Moyer , D. , Schilling , K. G. , Rheault , F. , & Landman , B. ( 2023. ). Convolutional-recurrent neural networks approximate diffusion tractography from t1-weighted MRI and associated anatomical context . bioRxiv , 2023-02. 10.1101/2023.02.25.530046 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
