Therapeutic effect and mechanism of gigantol on hyperuricemia
- PMID: 40801026
- PMCID: PMC12339328
- DOI: 10.3389/fendo.2025.1474808
Therapeutic effect and mechanism of gigantol on hyperuricemia
Abstract
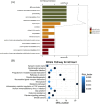
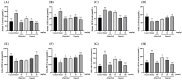
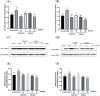
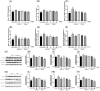
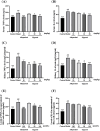
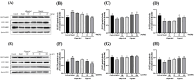
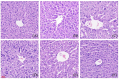
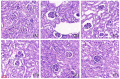
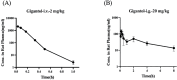
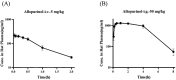
This study aimed to evaluate the therapeutic effects of gigantol on hyperuricemia (HUA) and investigate the underlying mechanism of HUA. A mouse model of HUA was made by gavage of potassium oxonate, and HK-2 and AML12 cell models were made by adenosine and xanthine oxidase (XOD) induction. We tested the levels of uric acid (UA), creatinine (CRE), blood urea nitrogen (BUN), cellular UA, and XOD activity. The levels of NOD-like receptor thermal protein domain 3 (NLRP3) and other inflammatory factors were detected by enzyme-linked immunosorbent assay (ELISA) kits. XOD is a protein related to the NLRP3 pathway and also serves as an UA transporter. We found that the levels of UA, CRE, and BUN increased in serum but decreased in urine in HUA model mice. After gigantol treatment, UA, CRE, and BUN levels in serum decreased, whereas their levels in urine increased. The levels of NLRP3 and interleukin-1β (IL-1β) were lower and the expression of NLRP3-related protein decreased after gigantol treatment. In conclusion, gigantol exhibits a therapeutic effect on HUA, and the mechanism may be related to inhibiting XOD activity to reduce UA production, regulating the expression of UA transporters to increase UA excretion, and inhibiting the activation of NLRP3 inflammatory signaling.
Keywords: HUA; NLRP3; UA transporters; gigantol; xanthine oxidase inhibitor.
Copyright © 2025 Wu, Sun, Jia, Gao, Xu, Qian, Pei, Wang, Zheng, Li, Chen, Liu, Ma, Chen, Ye, Zhao, Zhou, Chen, Huang, Liu, Zhu, Xue, Zhang, Ji and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Shanling Jiangzhi Tea treats hyperuricemia by inhibition of COMT/MAOA signaling pathway and p53/SERPINE1/NLRP3 signaling pathway.Front Endocrinol (Lausanne). 2025 Jul 11;16:1623111. doi: 10.3389/fendo.2025.1623111. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40717803 Free PMC article.
-
Combination of Withania coagulans and Fagonia cretica ameliorates hyperuricemia by re-modulating gut microbiota-derived spermidine and traumatic acid.Phytomedicine. 2025 Sep;145:157079. doi: 10.1016/j.phymed.2025.157079. Epub 2025 Jul 17. Phytomedicine. 2025. PMID: 40712280
-
Antihyperuricemic, hepatoprotective and nephroprotective roles of Benincasae Exocarpium in hyperuricemia rats.J Ethnopharmacol. 2025 Aug 29;352:120185. doi: 10.1016/j.jep.2025.120185. Epub 2025 Jun 20. J Ethnopharmacol. 2025. PMID: 40544980
-
[Association between gut microbiota and hyperuricemia: insights into innovative therapeutic strategies].Sheng Wu Gong Cheng Xue Bao. 2025 Jun 25;41(6):2290-2309. doi: 10.13345/j.cjb.250060. Sheng Wu Gong Cheng Xue Bao. 2025. PMID: 40550671 Review. Chinese.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
References
-
- Li L, Zhang Y, Zeng C. Update on the epidemiology, genetics, and therapeutic options of hyperuricemia. Am J Transl Res. (2020) 12:3167–81. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC7407685/, PMID: - PMC - PubMed
-
- Wang YZ, Zhou C, Zhu LJ, He XL, Li LZ, Zheng X, et al. Effects of macroporous resin extract of dendrobium officinale leaves in rats with hyperuricemia induced by fructose and potassium oxonate. Comb Chem High Throughput Screen. (2022) 25:1294–303. doi: 10.2174/1386207324666210528114345, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

