Copper ferrite-graphene oxide catalyst for enhanced peroxymonosulfate activation and pollutant degradation
- PMID: 40801044
- PMCID: PMC12336850
- DOI: 10.1039/d5na00409h
Copper ferrite-graphene oxide catalyst for enhanced peroxymonosulfate activation and pollutant degradation
Abstract
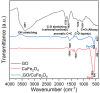
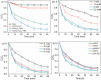
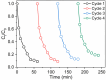
In this study, a magnetic nanocomposite of copper ferrite (CuFe2O4) supported on reduced graphene oxide (rGO) was synthesized via a solvothermal method and applied as a catalyst for the activation of peroxymonosulfate (PMS) to degrade Orange G (OG) dye in aqueous solution. The structure and morphology of the catalyst were thoroughly characterized using XRD, FTIR, SEM, STEM, and nitrogen adsorption-desorption analyses. The rGO/CuFe2O4 composite demonstrated superior catalytic performance, achieving 90.8% OG removal within 60 minutes, attributed to its enhanced surface area, efficient radical generation, and strong interaction between rGO and CuFe2O4. The system exhibited high activity across a wide pH range, significant mineralization (78% TOC removal), and good recyclability over four cycles. The catalyst also effectively degraded other dyes including rhodamine B (78%), methylene blue (86%), and methyl orange (89%) under similar conditions. These findings suggest that rGO/CuFe2O4 is a promising, reusable catalyst for advanced oxidation processes in wastewater treatment.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures










Similar articles
-
Efficient degradation of phenol by MnOOH-rGO composite with high peroxymonosulfate utilization efficiency.Chemosphere. 2023 Sep;336:139200. doi: 10.1016/j.chemosphere.2023.139200. Epub 2023 Jun 13. Chemosphere. 2023. PMID: 37321456
-
A New Powerful Magnetic Dark Catalyst Based on rGO/Fe3O4/CdSe Nanocomposite for Ultrafast Degradation of Methylene Blue Dye.J Fluoresc. 2025 Jul;35(7):5847-5859. doi: 10.1007/s10895-024-03982-5. Epub 2024 Oct 3. J Fluoresc. 2025. PMID: 39361191
-
Autonomous self-propelled reduced graphene oxide hydrogel-CuO@rGO micromotors for high-efficiency removal of organic dye pollutant.J Contam Hydrol. 2025 Sep;274:104650. doi: 10.1016/j.jconhyd.2025.104650. Epub 2025 Jun 13. J Contam Hydrol. 2025. PMID: 40532439
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
References
-
- Verma N. Chundawat T. S. Chandra H. Vaya D. Role of ZnO/GO Nanocomposite in Photocatalytic Degradation of Mixture of Organic Pollutants and Antimicrobial Activity. ChemistrySelect. 2024;9(3):e202303022. doi: 10.1002/SLCT.202303022. - DOI
-
- Lellis B. Fávaro-Polonio C. Z. Pamphile J. A. Polonio J. C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnol. Res. Innov. 2019;3(2):275–290. doi: 10.1016/J.BIORI.2019.09.001. - DOI
-
- Zhang Q. Peng Y. Deng F. Wang M. Chen D. Porous Z-Scheme MnO2/Mn-Modified Alkalinized g-C3N4 Heterojunction with Excellent Fenton-like Photocatalytic Activity for Efficient Degradation of Pharmaceutical Pollutants. Sep. Purif. Technol. 2020;246:116890. doi: 10.1016/J.SEPPUR.2020.116890. - DOI
-
- Talukdar S. Dutta R. K. A Mechanistic Approach for Superoxide Radicals and Singlet Oxygen Mediated Enhanced Photocatalytic Dye Degradation by Selenium Doped ZnS Nanoparticles. RSC Adv. 2015;6(2):928–936. doi: 10.1039/C5RA17940H. - DOI
-
- Mukherjee J. Lodh B. K. Sharma R. Mahata N. Shah M. P. Mandal S. Ghanta S. Bhunia B. Advanced Oxidation Process for the Treatment of Industrial Wastewater: A Review on Strategies, Mechanisms, Bottlenecks and Prospects. Chemosphere. 2023;345:140473. doi: 10.1016/J.CHEMOSPHERE.2023.140473. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

