Combined Effects of Metformin, Quercetin, and Fractionated Gamma Irradiation on MiR-107-Mediated Brain Injury in HFD/STZ-Induced Diabetic Rats
- PMID: 40801060
- PMCID: PMC12340211
- DOI: 10.1177/15593258251367627
Combined Effects of Metformin, Quercetin, and Fractionated Gamma Irradiation on MiR-107-Mediated Brain Injury in HFD/STZ-Induced Diabetic Rats
Abstract
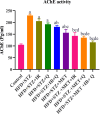
Objectives: MiR-107 upregulation represents a key target among the multiple pathways associated with T2DM. Given that drug combinations offer significant therapeutic potential, this study investigated the antidiabetic, antioxidant, and anti-inflammatory effects of γ-irradiation, the quercetin (common flavonol), and the metformin (biguanide) on HFD/STZ-induced diabetic rats' brains. Methods: Diabetic rats were treated with metformin (200 mg/kg b.w./day) alone or in combination with quercetin (30 mg/kg b.w./day) and/or γ-radiation (fractionated 4 Gy) for 4 weeks. Results: The diabetic group exhibited increased body weight, blood glucose, HOMA-IR, AChE, MMP-2, and lipid peroxidation, while serum insulin and brain GPx antioxidant enzyme activity were significantly decreased. Similarly, BDNF and SIRTI transcript levels and IRS1 protein expression were reduced, whereas NF-κB and MiR-107 transcript levels were elevated in diabetic rats compared to controls. Histopathological examination of diabetic brain tissue corroborated the biochemical findings. Treatment with metformin alone or in combination with γ-irradiation and/or quercetin effectively mitigated these effects by downregulating miR-107 and improving brain function, with optimal results achieved through combined therapy. Conclusion: The synergistic combination of Metformin, fractionated gamma-irradiation, and quercetin effectively attenuates brain injury in diabetic rats by enhancing IRS1/SIRT1/BDNF signaling while suppressing MiR-107/NF-κB pathways.
Keywords: BDNF; IRS1; MiR-107; NF-κB; SIRT1; biguanide.
© The Author(s) 2025.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures









Similar articles
-
Study on the modulation of kidney and liver function of rats with diabetic nephropathy by Huidouba through metabolomics.J Ethnopharmacol. 2025 Jul 24;351:120136. doi: 10.1016/j.jep.2025.120136. Epub 2025 Jun 11. J Ethnopharmacol. 2025. PMID: 40513925
-
Combined Metformin and Baricitinib Therapy Attenuates Inflammation in STZ-Induced Diabetic Rats via AMPK/JAK-STAT Pathway Crosstalk.Endocrinol Diabetes Metab. 2025 Sep;8(5):e70101. doi: 10.1002/edm2.70101. Endocrinol Diabetes Metab. 2025. PMID: 40903907 Free PMC article.
-
[Mechanism of Qingrun Decoction in alleviating hepatic insulin resistance in type 2 diabetic rats based on amino acid metabolism reprogramming pathways].Zhongguo Zhong Yao Za Zhi. 2025 Jun;50(12):3377-3388. doi: 10.19540/j.cnki.cjcmm.20250120.701. Zhongguo Zhong Yao Za Zhi. 2025. PMID: 40686115 Chinese.
-
NTP Developmental and Reproductive Toxicity Technical Report on the Prenatal Development Studies of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine (CASRN 95737-68-1) in Sprague Dawley (Hsd:Sprague Dawley® SD®) Rats and New Zealand White (Hra:NZW SPF) Rabbits: DART Report 07 [Internet].Research Triangle Park (NC): National Toxicology Program; 2022 Jan. Research Triangle Park (NC): National Toxicology Program; 2022 Jan. PMID: 35593777 Free Books & Documents. Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Bhattacharya S, Ahsan CR, Dutta P, et al. Metformin: beyond diabetes management to neuroprotection and cognitive enhancement. Curr Pharmacol Rep. 2025;11:1-18. doi: 10.1007/s40495-025-00395-1 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

