An Animal Model of Liuzijue Based on Kinematic Features Exploration: A Pilot Study Conducting in COPD
- PMID: 40801240
- PMCID: PMC12344581
- DOI: 10.1002/iid3.70233
An Animal Model of Liuzijue Based on Kinematic Features Exploration: A Pilot Study Conducting in COPD
Abstract
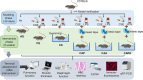
Background: Liuzijue is an essential nonpharmacological intervention within the comprehensive management strategies for chronic obstructive pulmonary disease (COPD) patients. However, the absence of an animal model for Liuzijue presents methodological limitations in its basic research. This study aimed to apply interventions with kinematic characteristics of Liuzijue to COPD mice, with the objective to explore an animal model of Liuzijue suitable for experimental studies.
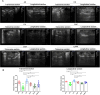
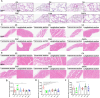
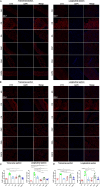
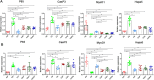
Methods: Forty-eight C57BL/6 mice randomly assigned into six groups to receive interventions mimicking Liuzijue's kinematic features, namely aerobic exercise, pursed-lip breathing and abdominal breathing. Post-intervention, respiratory function, diaphragmatic contractility, and rectus abdominis thickness were assessed. Histological structures of lung tissue, diaphragm, and rectus abdominis were observed using H&E staining. Expression levels of IL-10, INF-γ, and TNF-α in bronchoalveolar lavage fluid, and p65, CasP3, MuRF1, MyoD1, IGF-1, and Hspa5 in the diaphragm and rectus abdominis were measured.
Results: The modeling process impaired respiratory function and diaphragmatic contractility in mice. All four stimulation forms effectively improved pulmonary and diaphragmatic function in COPD mice. The thickness of the rectus abdominis was increased by three specified exercise forms. Despite minimal lung tissue structural changes, swimming and abdominal stimulation improved the structure of the rectus abdominis in COPD mice and airway inflammation levels were inhibited. Lastly, the four stimulations regulated the balance of myoprotein synthesis and degradation in the diaphragm and rectus abdominis, although the intervention effects of the four stimulations did not escalate with the complexity of the methods.
Conclusion: The exercise stimulation paradigms established by simulating the kinematic characteristics of Liuzijue possess the therapeutic effects of Liuzijue in improving respiratory function, demonstrating first evidence that kinematic-based animal models can bridge traditional Qigong and modern mechanism research. However, the development of an animal model for the application of Liuzijue in basic research still warrants further exploration.
Keywords: Liuzijue; animal model; chronic obstructive pulmonary disease; kinematic feature; pulmonary rehabilitation.
© 2025 The Author(s). Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Effects of different traditional Chinese exercises on pulmonary function in patients with stable chronic obstructive pulmonary disease: a network meta-analysis.BMC Complement Med Ther. 2024 Aug 14;24(1):304. doi: 10.1186/s12906-024-04609-9. BMC Complement Med Ther. 2024. PMID: 39143580 Free PMC article.
-
Efficacy of Liuzijue Qigong in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis.Complement Ther Med. 2022 May;65:102809. doi: 10.1016/j.ctim.2022.102809. Epub 2022 Jan 29. Complement Ther Med. 2022. PMID: 35093513
-
Pulmonary rehabilitation for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2015 Feb 23;2015(2):CD003793. doi: 10.1002/14651858.CD003793.pub3. Cochrane Database Syst Rev. 2015. PMID: 25705944 Free PMC article.
-
Characteristic activation of respiratory muscles in Liuzijue practice: a preliminary study based on surface electromyography in healthy individuals.Eur J Appl Physiol. 2025 Jul 28. doi: 10.1007/s00421-025-05915-x. Online ahead of print. Eur J Appl Physiol. 2025. PMID: 40721517
-
Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2017 May 23;5(5):CD011425. doi: 10.1002/14651858.CD011425.pub2. Cochrane Database Syst Rev. 2017. PMID: 28535331 Free PMC article.
References
-
- Pelgrim C. E., Peterson J. D., Gosker H. R., et al., “Psychological Co‐Morbidities in COPD: Targeting Systemic Inflammation, a Benefit for Both?,” European Journal of Pharmacology 842 (2019): 99–110. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

