Human Umbilical Cord Mesenchymal Stem Cells Modulate Cytokine Secretion of CD4+ T Cell in Systemic Lupus Erythematosus by Inhibiting HSP90AA1 in the Glucose-Activated PI3K-AKT Pathway
- PMID: 40801323
- PMCID: PMC12344575
- DOI: 10.1002/iid3.70239
Human Umbilical Cord Mesenchymal Stem Cells Modulate Cytokine Secretion of CD4+ T Cell in Systemic Lupus Erythematosus by Inhibiting HSP90AA1 in the Glucose-Activated PI3K-AKT Pathway
Abstract
Objective: Treatment with human umbilical cord mesenchymal stem cells (hUC-MSCs) attenuated the clinical manifestations of systemic lupus erythematosus (SLE). We investigated the metabolic mechanism whereby hUC-MSCs modify CD4+ T cell cytokine secretion in lupus.
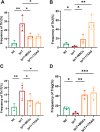
Methods: The study enrolled 30 untreated lupus patients and 20 sex, age, and body mass index matched healthy controls (HCs). CD4+ T cells were isolated by magnetic sorting, and stimulated with anti-CD3/CD28. The hUC-MSCs treatment (MSCT) groups were coculturing hUC-MSCs to CD4+ T cells from moderate and severe SLE (SLE-MS) groups for 72 h at ratios of 1:25 (T1), 1:10 (T2), and 1:5 (T3). Cytokine concentration and proliferation of the CD4+ T cells were measured by Luminex liquid chip assay and cell counting kit-8, respectively. Glucose metabolic capacity was measured by Seahorse real-time metabolic analysis. The role of hUC-MSCs on cytokine secretion was analyzed by transcriptome sequencing. Glucose enzymes levels and HSP90AA1/PI3K/AKT pathway activity were analyzed by real-time quantitative PCR and western blot. The CD4+ T cell subsets were detected by flow cytometry.
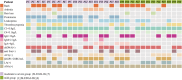
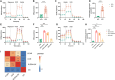
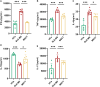
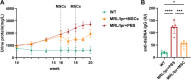
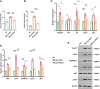
Results: Compared with HCs, the enhanced glycolysis and mitochondrial oxygen consumption of SLE-CD4+ T cells were positively associated with disease activity. Treatment with hUC-MSCs proportionally decreased glucose metabolism and proliferation of SLE-CD4+ T cells. The hUC-MSCs treatment significantly diminished supernatant concentrations of interferon-γ, tumor necrosis factor-α, interleukin (IL)-4, and IL-17 in SLE-MS group, as well as inhibited HSP90AA1 in the glucose-activated PI3K-AKT pathway. In animal experiment, the systemic administration of hUC-MSCs and inhibition of HSP90AA1 resulted in a reduction of glucose metabolites, enzymes, pro-inflammatory factor levels, and HSP90AA1/PI3K/AKT signaling pathway activity.
Conclusions: The hUC-MSCs treatment inhibited overactive glucose metabolism of SLE-CD4+ T cells. HSP90AA1 in the PI3K-AKT pathway induced by the glucose metabolism may be involved in the anti-inflammatory function of hUC-MSCs treatment.
Keywords: CD4+ T cell; HSP90AA1; Lupus; PI3K‐AKT pathway; cytokine; glucose metabolism; mesenchymal stem cell.
© 2025 The Author(s). Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








Similar articles
-
Human Umbilical Cord Mesenchymal Stem Cells for the Treatment of Systemic Lupus Erythematosus via Glucose Metabolism of CD4+T Cells.Stem Cell Rev Rep. 2025 May;21(4):1013-1033. doi: 10.1007/s12015-025-10848-1. Epub 2025 Feb 20. Stem Cell Rev Rep. 2025. PMID: 39976800 Free PMC article.
-
The proliferation/migration ability mediated by CD151/PI3K/AKT pathway determines the therapeutic effect of hUC-MSCs transplantation on rheumatoid arthritis.Clin Exp Hypertens. 2024 Dec 31;46(1):2366270. doi: 10.1080/10641963.2024.2366270. Epub 2024 Jun 12. Clin Exp Hypertens. 2024. PMID: 38864268
-
Leptin and Neutrophil-Activating Peptide 2 Promote Mesenchymal Stem Cell Senescence Through Activation of the Phosphatidylinositol 3-Kinase/Akt Pathway in Patients With Systemic Lupus Erythematosus.Arthritis Rheumatol. 2015 Sep;67(9):2383-93. doi: 10.1002/art.39196. Arthritis Rheumatol. 2015. PMID: 25989537 Free PMC article.
-
Cell Sheets Formation Enhances Therapeutic Effects of Human Umbilical Cord Mesenchymal Stem Cells on Spinal Cord Injury.CNS Neurosci Ther. 2024 Dec;30(12):e70163. doi: 10.1111/cns.70163. CNS Neurosci Ther. 2024. PMID: 39670537 Free PMC article.
-
Mesenchymal stem cell transplantation may be able to induce immunological tolerance in systemic lupus erythematosus.Biomed J. 2024 Aug;47(4):100724. doi: 10.1016/j.bj.2024.100724. Epub 2024 Apr 13. Biomed J. 2024. PMID: 38616015 Free PMC article. Review.
References
-
- Li H., Boulougoura A., Endo Y., and Tsokos G. C., “Abnormalities of T Cells in Systemic Lupus Erythematosus: New Insights in Pathogenesis and Therapeutic Strategies,” Journal of Autoimmunity 132 (2022): 102870. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

