The mRNA Translation Inhibitor Vioprolide A Prevents Inflammatory Pain-Like Behaviour With Limited Action on Already Established Pain-Like Behaviour in Mice
- PMID: 40801493
- PMCID: PMC12345401
- DOI: 10.1002/ejp.70099
The mRNA Translation Inhibitor Vioprolide A Prevents Inflammatory Pain-Like Behaviour With Limited Action on Already Established Pain-Like Behaviour in Mice
Abstract
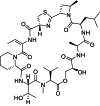
Background: Accumulating evidence indicates that pharmacological inhibition of the translational machinery is a therapeutic strategy for various diseases. However, whether inhibitors of mRNA translation might be suitable for pain therapy remains poorly understood. Here, we tested the potential analgesic effects of the natural product vioprolide A, which targets nucleolar protein 14 (NOP14) that is essential for ribosome biogenesis, in mouse models of pain.
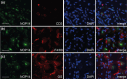
Methods: We assessed the antinociceptive effects of vioprolide A in C57BL/6 mice using four different models: zymosan-induced peritonitis, zymosan-induced paw inflammation, complete Freund's adjuvant-induced paw inflammation and spared nerve injury. Plasma and brain levels of vioprolide A were determined in a pharmacokinetic study. Immunostaining and western blot experiments were performed to investigate the distribution and expression of NOP14 in dorsal root ganglia.
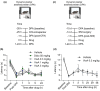
Results: Pretreatment with vioprolide A alleviated the visceral inflammatory hypersensitivity during zymosan-induced peritonitis, and it attenuated the somatic inflammatory hypersensitivity during zymosan-induced paw inflammation in a dose-dependent manner. However, treatment with vioprolide A did not affect established hypersensitivities. Pharmacokinetic measurements revealed that vioprolide A was not brain-penetrant and exhibited a short plasma half-life, which however seems to be sufficient to exert long-lasting antinociceptive effects. Tissue stainings revealed that NOP14 is expressed in a population of sensory neurons.
Conclusions: Our findings imply that vioprolide A may alleviate inflammatory nociceptive behaviours, but highlight that these effects may be limited to specific types of pain and treatment strategies.
Significance statement: The inhibitor of mRNA translation, vioprolide A, produced robust antinociception in distinct murine models of pain. This study provides evidence supporting further investigation of mRNA translation inhibitors, which attenuate pain by a novel mechanism of action that is not shared by established analgesics.
© 2025 The Author(s). European Journal of Pain published by John Wiley & Sons Ltd on behalf of European Pain Federation ‐ EFIC ®.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Oxycodone for cancer-related pain.Cochrane Database Syst Rev. 2022 Jun 9;6(6):CD003870. doi: 10.1002/14651858.CD003870.pub7. Cochrane Database Syst Rev. 2022. PMID: 35679121 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
-
- Balzulat, A. , Zhu W. F., Flauaus C., et al. 2024. “Discovery of a Small Molecule Activator of Slack (Kcnt1) Potassium Channels That Significantly Reduces Scratching in Mouse Models of Histamine‐Independent and Chronic Itch.” Advanced Science 11: e2307237. 10.1002/advs.202307237. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

