Prosaposin: A Multifaceted Protein Orchestrating Biological Processes and Diseases
- PMID: 40801564
- PMCID: PMC12345702
- DOI: 10.3390/cells14151131
Prosaposin: A Multifaceted Protein Orchestrating Biological Processes and Diseases
Abstract
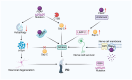
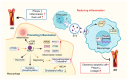
Prosaposin (PSAP), a multifunctional protein, plays a central role in various biological processes and diseases. It is the precursor of lysosomal activating protein, which is important for lipid metabolism and glucose metabolism. PSAP is implicated in cell signaling, neuroprotection, immunomodulation, and tumorigenesis. In neurological disorders, PSAP acts as a neurotrophic factor influencing nerve cell survival and synapse growth, and its dysfunction is associated with a variety of diseases. It modulates immune responses and macrophage functions, affecting inflammation and immune cell activities. The role of PSAP in cancers is complex, because it promotes or inhibits tumor growth depending on the context and it serves as a potential biomarker for various malignancies. This review examines current research on the functional and pathological roles of PSAP, emphasizing the importance of PSAP in Gaucher disease, neurodegenerative diseases, cardiovascular diseases, and cancer. In order to develop targeted therapies for various diseases, it is essential to understand the mechanisms of action of PSAP in different biological processes.
Keywords: Alzheimer’s disease; Gaucher disease; Parkinson’s disease; atherosclerosis; cancer; prosaposin; saposins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
The Two Faces of HDAC3: Neuroinflammation in Disease and Neuroprotection in Recovery.Epigenomics. 2024 Nov-Nov;16(21-22):1373-1388. doi: 10.1080/17501911.2024.2419357. Epub 2024 Nov 8. Epigenomics. 2024. PMID: 39513228 Review.
-
Expression patterns of prosaposin and its receptors, G protein-coupled receptor (GPR) 37 and GPR37L1 mRNAs, in the chick inner ear.Cell Tissue Res. 2023 May;392(2):481-497. doi: 10.1007/s00441-023-03753-x. Epub 2023 Feb 8. Cell Tissue Res. 2023. PMID: 36750499
-
Comprehensive Insights Into Mitophagy: Mechanisms, Disease Associations, and Therapeutic Implications.J Cell Biochem. 2025 Jul;126(7):e70056. doi: 10.1002/jcb.70056. J Cell Biochem. 2025. PMID: 40714832 Review.
-
Genistein - A Broad-spectrum Bioactive Compound with Diverse Pharmacological Potential: A Systematic Review.Curr Mol Med. 2025 Jul 24. doi: 10.2174/0115665240377727250703130718. Online ahead of print. Curr Mol Med. 2025. PMID: 40734425
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
References
-
- Kang S.J., Halvorsen O.J., Gravdal K., Bhattacharya N., Lee J.M., Liu N.W., Johnston B.T., Johnston A.B., Haukaas S.A., Aamodt K., et al. Prosaposin inhibits tumor metastasis via paracrine and endocrine stimulation of stromal p53 and Tsp-1. Proc. Natl. Acad. Sci. USA. 2009;106:12115–12120. doi: 10.1073/pnas.0903120106. Erratum in Proc. Natl. Acad. Sci. USA 2023, 120, e2320128120. https://doi.org/10.1073/pnas.2320128120 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

