Long-Term Engraftment and Satellite Cell Expansion from Human PSC Teratoma-Derived Myogenic Progenitors
- PMID: 40801582
- PMCID: PMC12345712
- DOI: 10.3390/cells14151150
Long-Term Engraftment and Satellite Cell Expansion from Human PSC Teratoma-Derived Myogenic Progenitors
Abstract
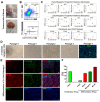
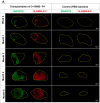
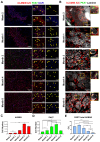
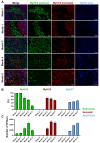
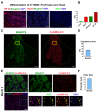
Skeletal muscle regeneration requires a reliable source of myogenic progenitor cells capable of forming new fibers and creating a self-renewing satellite cell pool. Human induced pluripotent stem cell (hiPSC)-derived teratomas have emerged as a novel in vivo platform for generating skeletal myogenic progenitors, although in vivo studies to date have provided only an early single-time-point snapshot. In this study, we isolated a specific population of CD82+ ERBB3+ NGFR+ cells from human iPSC-derived teratomas and verified their long-term in vivo regenerative capacity following transplantation into NSG-mdx4Cv mice. Transplanted cells engrafted, expanded, and generated human Dystrophin+ muscle fibers that increased in size over time and persisted stably long-term. A dynamic population of PAX7+ human satellite cells was established, initially expanding post-transplantation and declining moderately between 4 and 8 months as fibers matured. MyHC isoform analysis revealed a time-based shift from embryonic to neonatal and slow fiber types, indicating a slow progressive maturation of the graft. We further show that these progenitors can be cryopreserved and maintain their engraftment potential. Together, these findings give insight into the evolution of teratoma-derived human myogenic stem cell grafts, and highlight the long-term regenerative potential of teratoma-derived human skeletal myogenic progenitors.
Keywords: PAX7; iPS cells; regeneration; satellite cells; skeletal muscle; teratoma; transplantation; xenograft.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

