Molecular Insights into the Superiority of Platelet Lysate over FBS for hASC Expansion and Wound Healing
- PMID: 40801587
- PMCID: PMC12346565
- DOI: 10.3390/cells14151154
Molecular Insights into the Superiority of Platelet Lysate over FBS for hASC Expansion and Wound Healing
Abstract
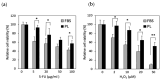
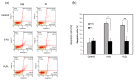
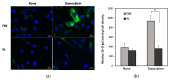
Human adipose-derived stem cells (hASCs) are widely used in regenerative medicine due to their accessibility and high proliferative capacity. Platelet lysate (PL) has recently emerged as a promising alternative to fetal bovine serum (FBS), offering superior cell expansion potential; however, the molecular basis for its efficacy remains insufficiently elucidated. In this study, we performed RNA sequencing to compare hASCs cultured with PL or FBS, revealing a significant upregulation of genes related to stress response and cell proliferation under PL conditions. These findings were validated by RT-qPCR and supported by functional assays demonstrating enhanced cellular resilience to oxidative and genotoxic stress, reduced doxorubicin-induced senescence, and improved antiapoptotic properties. In a murine wound model, PL-treated wounds showed accelerated healing, characterized by thicker dermis-like tissue formation and increased angiogenesis. Immunohistochemical analysis further revealed elevated expression of chk1, a DNA damage response kinase encoded by CHEK1, which plays a central role in maintaining genomic integrity during stress-induced repair. Collectively, these results highlight PL not only as a viable substitute for FBS in hASC expansion but also as a bioactive supplement that enhances regenerative efficacy by promoting proliferation, stress resistance, and antiaging functions.
Keywords: RNA sequencing; antiaging effects; cell proliferation; human adipose-derived stem cells; platelet lysate; stress resistance.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the study’s design; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

