Impaired Long-Term Quantitative Cellular Response to SARS-CoV-2 Vaccine in Thiopurine-Treated IBD Patients
- PMID: 40801589
- PMCID: PMC12346008
- DOI: 10.3390/cells14151156
Impaired Long-Term Quantitative Cellular Response to SARS-CoV-2 Vaccine in Thiopurine-Treated IBD Patients
Abstract
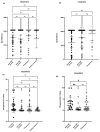
Background: Studies investigating the long-term cellular immune response to SARS-CoV-2 mRNA vaccines in patients with inflammatory bowel disease (IBD) remain limited, particularly among those receiving immunosuppressive therapy. Methods: We prospectively evaluated humoral and cellular immune responses at short-term (4-6 weeks) and long-term (6-12 months) time points following SARS-CoV-2 mRNA vaccination in patients with IBD receiving anti-TNF agents, thiopurines, or combination therapy. We defined the short-term response as the measurement taken 4-6 weeks after the second vaccine dose and the long-term response as the measurement taken between 6 and 12 months after the first determination. A cohort of healthy controls was included for short-term comparative analysis. Results: At long-term follow-up, quantitative humoral responses were reduced in patients receiving anti-TNF monotherapy. In contrast, a reduced quantitative cellular response was found in the thiopurine (median 0.7 UI/mL, p < 0.05) and anti-TNF combo groups (median 0.4 UI/mL, p < 0.01) compared to anti-TNF monotherapy (median 2.2 UI/mL). Conclusions: There was a robust long-term humoral and cellular response to vaccination, but a diminished quantitative cellular response in patients treated with thiopurines or combo therapy compared to anti-TNF monotherapy.
Keywords: SARS-CoV2 vaccine; cellular response; inflammatory bowel disease; thiopurines.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Alexander J.L., A Kennedy N., Ibraheim H., Anandabaskaran S., Saifuddin A., Seoane R.C., Liu Z., Nice R., Bewshea C., D’MEllo A., et al. COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): A multicentre, prospective, case-control study. Lancet Gastroenterol. Hepatol. 2022;7:342–352. doi: 10.1016/S2468-1253(22)00005-X. - DOI - PMC - PubMed
-
- Kennedy N.A., Lin S., Goodhand J.R., Chanchlani N., Hamilton B., Bewshea C., Nice R., Chee D., Cummings J.F., Fraser A., et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut. 2021;70:1884–1893. doi: 10.1136/gutjnl-2021-324789. - DOI - PubMed
-
- Alexander J.L., Liu Z., Sandoval D.M., Reynolds C., Ibraheim H., Anandabaskaran S., Saifuddin A., Seoane R.C., Anand N., Nice R., et al. COVID-19 vaccine-induced antibody and T-cell responses in immunosuppressed patients with inflammatory bowel disease after the third vaccine dose (VIP): A multicentre, prospective, case-control study. Lancet Gastroenterol. Hepatol. 2022;7:1005–1015. doi: 10.1016/S2468-1253(22)00274-6. - DOI - PMC - PubMed
-
- Lin S., Kennedy N.A., Saifuddin A., Sandoval D.M., Reynolds C.J., Seoane R.C., Kottoor S.H., Pieper F.P., Lin K.-M., Butler D.K., et al. Antibody decay, T cell immunity and breakthrough infections following two SARS-CoV-2 vaccine doses in inflammatory bowel disease patients treated with infliximab and vedolizumab. Nat. Commun. 2022;13:1–14. doi: 10.1038/s41467-022-28517-z. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

