Pilot Study of [11C]HY-2-15: A Mixed Alpha-Synuclein and Tau PET Radiotracer
- PMID: 40801590
- PMCID: PMC12346455
- DOI: 10.3390/cells14151157
Pilot Study of [11C]HY-2-15: A Mixed Alpha-Synuclein and Tau PET Radiotracer
Abstract
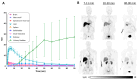
A novel brain positron emission tomography (PET) radioligand, [11C]HY-2-15, has potential for imaging alpha-synuclein aggregations in multiple system atrophy and misfolded tau proteins in tauopathies, based on its high binding affinity in disease brain tissue homogenates. Here, we demonstrate that [3H]HY-2-15 has the capability to bind to aggregated alpha-synuclein in multiple system atrophy brain and tau aggregations in progressive supranuclear palsy and corticobasal degeneration brain tissues via in vitro autoradiography study. A first-in-human pilot multicenter clinical study recruited a total of 10 subjects including healthy controls and patients with Parkinson's disease, multiple system atrophy, or progressive supranuclear palsy. The study revealed that [11C]HY-2-15 has a relatively higher specific uptake in the pallidum and midbrain of patients with progressive supranuclear palsy. Total-body scans performed on the PennPET Explorer showed the radiotracer was cleared by renal excretion. However, the rapid metabolism and low brain uptake resulted in a limited signal of [11C]HY-2-15 in brain.
Keywords: PET; Parkinson’s disease; multiple system atrophy; progressive supranuclear palsy; radioligand; tau; α-synuclein.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

