Parkinson's Disease: Bridging Gaps, Building Biomarkers, and Reimagining Clinical Translation
- PMID: 40801594
- PMCID: PMC12346353
- DOI: 10.3390/cells14151161
Parkinson's Disease: Bridging Gaps, Building Biomarkers, and Reimagining Clinical Translation
Abstract
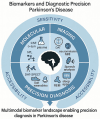
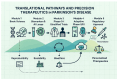
Parkinson's disease (PD), a progressive neurodegenerative disorder, imposes growing clinical and socioeconomic burdens worldwide. Despite landmark discoveries in dopamine biology and α-synuclein pathology, translating mechanistic insights into effective, personalized interventions remains elusive. Recent advances in molecular profiling, neuroimaging, and computational modeling have broadened the understanding of PD as a multifactorial systems disorder rather than a purely dopaminergic condition. However, critical gaps persist in diagnostic precision, biomarker standardization, and the translation of bench side findings into clinically meaningful therapies. This review critically examines the current landscape of PD research, identifying conceptual blind spots and methodological shortfalls across pathophysiology, clinical evaluation, trial design, and translational readiness. By synthesizing evidence from molecular neuroscience, data science, and global health, the review proposes strategic directions to recalibrate the research agenda toward precision neurology. Here I highlight the urgent need for interdisciplinary, globally inclusive, and biomarker-driven frameworks to overcome the fragmented progression of PD research. Grounded in the Accelerating Medicines Partnership-Parkinson's Disease (AMP-PD) and the Parkinson's Progression Markers Initiative (PPMI), this review maps shared biomarkers, open data, and patient-driven tools to faster personalized treatment. In doing so, it offers actionable insights for researchers, clinicians, and policymakers working at the intersection of biology, technology, and healthcare delivery. As the field pivots from symptomatic relief to disease modification, the road forward must be cohesive, collaborative, and rigorously translational, ensuring that laboratory discoveries systematically progress to clinical application.
Keywords: Parkinson’s disease diagnosis; Parkinson’s disease therapy; artificial intelligence applications; biomarkers analysis; clinical trials standards; communication; global health trends; neurodegenerative diseases pathophysiology; precision-medicine methods; translational medical research methods.
Conflict of interest statement
The author declares no conflicts of interest.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
SynNeurGe: The road ahead for a biological definition of Parkinson's disease.J Parkinsons Dis. 2025 Aug;15(5):931-938. doi: 10.1177/1877718X241298194. Epub 2024 Dec 8. J Parkinsons Dis. 2025. PMID: 39973492 Review.
-
MarkVCID cerebral small vessel consortium: I. Enrollment, clinical, fluid protocols.Alzheimers Dement. 2021 Apr;17(4):704-715. doi: 10.1002/alz.12215. Epub 2021 Jan 21. Alzheimers Dement. 2021. PMID: 33480172 Free PMC article.
-
Tissue Factor and Its Cerebrospinal Fluid Protein Profiles in Parkinson's Disease.J Parkinsons Dis. 2024;14(7):1405-1416. doi: 10.3233/JPD-240115. J Parkinsons Dis. 2024. PMID: 39240648 Free PMC article.
-
Addressing the need for standardization of symptomatic medication documentation in Parkinson's disease clinical research: A call to action.J Parkinsons Dis. 2025 Feb;15(1):227-235. doi: 10.1177/1877718X241305711. Epub 2025 Jan 14. J Parkinsons Dis. 2025. PMID: 39973486 Review.
References
-
- Waleed M., Sadiq W., Subhan S. Parkinsonism and D-512, dopamine D2/3 receptor agonist; A review of literature. Inter-Natl. J. Clin. Case Rep. Rev. 2020;4 doi: 10.31579/2690-4861/077. - DOI
-
- Scorza F.A., Menezes-Rodrigues F.S., Olszewer E., Errante P.R., Tavares J.G.P., Scorza C.A., Ferraz H.B., Finsterer J., Caricati-Neto A. The mitochondrial calcium uniporter: A new therapeutic target for Parkinson’s disease-related cardiac dysfunctions? Clinics. 2020;75:e1299. doi: 10.6061/clinics/2020/e1299. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

