Chaperone-Mediated Responses and Mitochondrial-Endoplasmic Reticulum Coupling: Emerging Insight into Alzheimer's Disease
- PMID: 40801612
- PMCID: PMC12346265
- DOI: 10.3390/cells14151179
Chaperone-Mediated Responses and Mitochondrial-Endoplasmic Reticulum Coupling: Emerging Insight into Alzheimer's Disease
Abstract
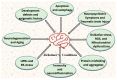
Alzheimer's disease (AD) is increasingly recognized as a multifactorial disorder driven by a combination of disruptions in proteostasis and organelle communication. The 2020 Lancet commission reported that approximately 10 million people worldwide were affected by AD in the mid-20th century. AD is the most prevalent cause of dementia. By early 2030, the global cost of dementia is projected to rise by USD 2 trillion per year, with up to 85% of that cost attributed to daily patient care. Several factors have been implicated in the progression of neurodegeneration, including increased oxidative stress, the accumulation of misfolded proteins, the formation of amyloid plaques and aggregates, the unfolded protein response (UPR), and mitochondrial-endoplasmic reticulum (ER) calcium homeostasis. However, the exact triggers that initiate these pathological processes remain unclear, in part because clinical symptoms often emerge gradually and subtly, complicating early diagnosis. Among the early hallmarks of neurodegeneration, elevated levels of reactive oxygen species (ROS) and the buildup of misfolded proteins are believed to play pivotal roles in disrupting proteostasis, leading to cognitive deficits and neuronal cell death. The accumulation of amyloid-β (Aβ) plaques and tau neurofibrillary tangles is a characteristic feature of AD. These features contribute to chronic neuroinflammation, which is marked by the release of pro-inflammatory cytokines and chemokines that exacerbate oxidative stress. Given these interconnected mechanisms, targeting stress-related signaling pathways, such as oxidative stress (ROS) generated in the mitochondria and ER, ER stress, UPR, and cytosolic chaperones, represents a promising strategy for therapeutic intervention. This review focuses on the relationship between stress chaperone responses and organelle function, particularly the interaction between mitochondria and the ER, in the development of new therapies for AD and related neurodegenerative disorders.
Keywords: Alzheimer’s disease; aggregates; amyloid-β; calcium; chaperones; endoplasmic reticulum; mitochondria.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
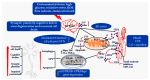
 )
indicates the expression of upregulated proteins, (
)
indicates the expression of upregulated proteins, ( )
denotes inhibitory activity, and (
)
denotes inhibitory activity, and ( ) represent the dynamics of Ca2+
ions within the pathways associated with ER and mitochondria.
) represent the dynamics of Ca2+
ions within the pathways associated with ER and mitochondria.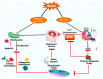
 ) indicate the expression of
upregulated proteins, (
) indicate the expression of
upregulated proteins, ( )
denotes inhibitory activity of the compounds.
)
denotes inhibitory activity of the compounds.Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Protein-rich foods, sea foods, and gut microbiota amplify immune responses in chronic diseases and cancers - Targeting PERK as a novel therapeutic strategy for chronic inflammatory diseases, neurodegenerative disorders, and cancer.Pharmacol Ther. 2024 Mar;255:108604. doi: 10.1016/j.pharmthera.2024.108604. Epub 2024 Feb 13. Pharmacol Ther. 2024. PMID: 38360205 Free PMC article. Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Recent advances in the detection and management of motor dysfunction in Alzheimer's disease.Psychiatriki. 2025 Jul 2;36(2):97-100. doi: 10.22365/jpsych.2025.012. Epub 2025 May 14. Psychiatriki. 2025. PMID: 40400272 English, Greek, Modern.
-
Phytocompounds as modulators of HSF1 in neurodegenerative disease: Special emphasis on Alzheimer's disease.Eur J Pharmacol. 2025 Aug 5;1005:178034. doi: 10.1016/j.ejphar.2025.178034. Online ahead of print. Eur J Pharmacol. 2025. PMID: 40769451 Review.
References
-
- Cummings J. Rarer dementias, limited options, and unaddressed needs: Commentary on “A Mixed Methods Evaluation of a Programme Exploring Pre-death Grief and Loss for Carers of People with Rarer Dementias,” by Stevens-Neck et al. Int. Psychogeriatr. 2024;36:451–454. doi: 10.1017/S1041610223000431. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

