Specific Low/Endogenous Replication Stress Response Protects Genomic Stability via Controlled ROS Production in an Adaptive Way and Is Dysregulated in Transformed Cells
- PMID: 40801615
- PMCID: PMC12346651
- DOI: 10.3390/cells14151183
Specific Low/Endogenous Replication Stress Response Protects Genomic Stability via Controlled ROS Production in an Adaptive Way and Is Dysregulated in Transformed Cells
Abstract
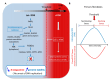
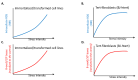
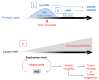
Cells are assaulted daily by stresses that jeopardize genome integrity. Primary human cells adapt their response to the intensity of replication stress (RS) in a diphasic manner: below a stress threshold, the canonical DNA damage response (cDDR) is not activated, but a noncanonical cellular response, low-level stress-DDR (LoL-DDR), has recently been described. LoL-DDR prevents the accumulation of premutagenic oxidized bases (8-oxoguanine) through the production of ROS in an adaptive way. The production of RS-induced ROS (RIR) is tightly controlled: RIR are excluded from the nucleus and are produced by the NADPH oxidases DUOX1/DUOX2, which are controlled by NF-κB and PARP1; then, RIR activate the FOXO1-detoxifying pathway. Increasing the intensity of RS suppresses RIR via p53 and ATM. Notably, LoL-DDR is dysregulated in cancer cell lines, in which RIR are not produced by NADPH oxidases, are not detoxified under high-level stress, and favor the accumulation of 8-oxoguanine. LoL-DDR dysregulation occurred at an early stage of cancer progression in an in vitro model. Since, conversely, ROS trigger RS, this establishes a vicious cycle that continuously jeopardizes genome integrity, fueling tumorigenesis. These data reveal a novel type of ROS-controlled DNA damage response and demonstrate the fine-tuning of the cellular response to stress. The effects on genomic stability and carcinogenesis are discussed here.
Keywords: DNA damage response; NF-κB; PARP1; ROS; genetic instability; replication stress.
Conflict of interest statement
The author declares having no conflict of interest.
Figures
Similar articles
-
Dysregulation of the low-level replication stress response in transformed cell lines.Sci Rep. 2025 Jun 6;15(1):20013. doi: 10.1038/s41598-025-05172-0. Sci Rep. 2025. PMID: 40481239 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
A noncanonical response to replication stress protects genome stability through ROS production, in an adaptive manner.Cell Death Differ. 2023 May;30(5):1349-1365. doi: 10.1038/s41418-023-01141-0. Epub 2023 Mar 3. Cell Death Differ. 2023. PMID: 36869180 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
References
-
- Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

