Structure of the Secretory Compartments in Goblet Cells in the Colon and Small Intestine
- PMID: 40801617
- PMCID: PMC12346421
- DOI: 10.3390/cells14151185
Structure of the Secretory Compartments in Goblet Cells in the Colon and Small Intestine
Abstract
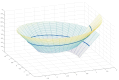
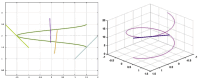
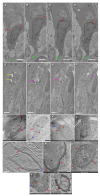
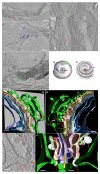
The Golgi of goblet cells represents a specialized machine for mucin glycosylation. This process occurs in a specialized form of the secretory pathway, which remains poorly examined. Here, using high-resolution three-dimensional electron microscopy (EM), EM tomography, serial block face scanning EM (SBF-SEM) and immune EM we analyzed the secretory pathway in goblet cells and revealed that COPII-coated buds on the endoplasmic reticulum (ER) are extremely rare. The ERES vesicles with dimensions typical for the COPII-dependent vesicles were not found. The Golgi is formed by a single cisterna organized in a spiral with characteristics of the cycloid surface. This ribbon has a shape of a cup with irregular perforations. The Golgi cup is filled with secretory granules (SGs) containing glycosylated mucins. Their diameter is close to 1 µm. The cup is connected with ER exit sites (ERESs) with temporal bead-like connections, which are observed mostly near the craters observed at the externally located cis surface of the cup. The craters represent conus-like cavities formed by aligned holes of gradually decreasing diameters through the first three Golgi cisternae. These craters are localized directly opposite the ERES. Clusters of the 52 nm vesicles are visible between Golgi cisternae and between SGs. The accumulation of mucin, started in the fourth cisternal layer, induces distensions of the cisternal lumen. The thickness of these distensions gradually increases in size through the next cisternal layers. The spherical distensions are observed at the edges of the Golgi cup, where they fuse with SGs and detach from the cisternae. After the fusion of SGs located just below the apical plasma membrane (APM) with APM, mucus is secreted. The content of this SG becomes less osmiophilic and the excessive surface area of the APM is formed. This membrane is eliminated through the detachment of bubbles filled with another SG and surrounded with a double membrane or by collapse of the empty SG and transformation of the double membrane lacking a visible lumen into multilayered organelles, which move to the cell basis and are secreted into the intercellular space where the processes of dendritic cells are localized. These data are evaluated from the point of view of existing models of intracellular transport.
Keywords: ER exit site; Golgi complex; Mucin; diffusion model; goblet cell; intracellular transport; kiss-and-run model; multilamellar organelle; regulation secretion; secretory granule.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
On the nature of the plant ER exit sites.Front Plant Sci. 2022 Oct 7;13:1010569. doi: 10.3389/fpls.2022.1010569. eCollection 2022. Front Plant Sci. 2022. PMID: 36275575 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
-
- Johansson M.E.V., Gustafsson J.K., Holmen-Larsson J., Jabbar K.S., Xia L., Xu H., Ghishan F.K., Carvalho F.A., Gewirtz A.T., Sjovall H., et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and in patients with ulcerative colitis. Gut. 2014;63:281–291. doi: 10.1136/gutjnl-2012-303207. - DOI - PMC - PubMed
-
- Pelaseyed T., Bergström J.H., Gustafsson J.K., Ermund A., Birchenough G.M., Schütte A., van der Post S., Svensson F., Rodríguez-Piñeiro A.M., Nyström E.E., et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014;260:8–20. doi: 10.1111/imr.12182. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

