Mechanistic Insights into the Pathogenesis of Polycystic Kidney Disease
- PMID: 40801635
- PMCID: PMC12345701
- DOI: 10.3390/cells14151203
Mechanistic Insights into the Pathogenesis of Polycystic Kidney Disease
Abstract
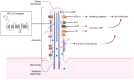
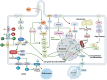
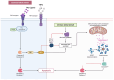
Autosomal Dominant Polycystic Kidney Disease (ADPKD) is a systemic ciliopathy resulting from loss-of-function mutations in the PKD1 and PKD2 genes, which encode polycystin-1 (PC1) and polycystin-2 (PC2), respectively. PC1 and PC2 regulate mechanosensation, calcium signaling, and key pathways controlling tubular epithelial structure and function. Loss of PC1/PC2 disrupts calcium homeostasis, elevates cAMP, and activates proliferative cascades such as PKA-B-Raf-MEK-ERK, mTOR, and Wnt, driving cystogenesis via epithelial proliferation, impaired apoptosis, fluid secretion, and fibrosis. Recent evidence also implicates novel signaling axes in ADPKD progression including, the Hippo pathway, where dysregulated YAP/TAZ activity enhances c-Myc-mediated proliferation; the stimulator of interferon genes (STING) pathway, which is activated by mitochondrial DNA release and linked to NF-κB-driven inflammation and fibrosis; and the TWEAK/Fn14 pathway, which mediates pro-inflammatory and pro-apoptotic responses via ERK and NF-κB activation in tubular cells. Mitochondrial dysfunction, oxidative stress, and maladaptive extracellular matrix remodeling further exacerbate disease progression. A refined understanding of ADPKD's complex signaling networks provides a foundation for precision medicine and next-generation therapeutics. This review gathers recent molecular insights and highlights both established and emerging targets to guide targeted treatment strategies in ADPKD.
Keywords: ADPKD; PC1/PC2; PKD; Wnt; apoptosis; cAMP; calcium; cilia; cystogenesis; fibrosis; mTOR; mitochondria.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Aung T.T., Bhandari S.K., Chen Q., Malik F.T., Willey C.J., Reynolds K., Jacobsen S.J., Sim J.J. Autosomal Dominant Polycystic Kidney Disease Prevalence among a Racially Diverse United States Population, 2002 through 2018. Kidney360. 2021;2:2010–2015. doi: 10.34067/KID.0004522021. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

