Residual Tumor Resection After Anti-PD-1 Therapy: A Promising Treatment Strategy for Overcoming Immune Evasive Phenotype Induced by Anti-PD-1 Therapy in Gastric Cancer
- PMID: 40801643
- PMCID: PMC12346003
- DOI: 10.3390/cells14151212
Residual Tumor Resection After Anti-PD-1 Therapy: A Promising Treatment Strategy for Overcoming Immune Evasive Phenotype Induced by Anti-PD-1 Therapy in Gastric Cancer
Abstract
Background: Anti-programmed death 1 receptor (PD-1) therapy is a promising treatment strategy for patients with unresectable advanced or recurrent gastric/gastroesophageal junction (G/GEJ) cancer. However, its response rate and survival benefits are still limited; an immunological analysis of the residual tumor after anti-PD-1 therapy would be important.
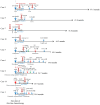
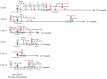
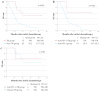
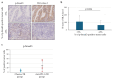
Methods: We evaluated the clinical efficacy of tumor resection (TR) after chemotherapy or anti-PD-1 therapy in patients with unresectable advanced or recurrent G/GEJ cancer and analyzed the immune status of tumor microenvironment (TME) by immunohistochemistry using their surgically resected specimens.
Results: Patients treated with TR after anti-PD-1 therapy had significantly longer survival compared to those treated with chemotherapy and anti-PD-1 therapy alone. Expression of human leukocyte antigen (HLA) class I and major histocompatibility complex (MHC) class II on tumor cells was markedly downregulated after anti-PD-1 therapy compared to chemotherapy. Furthermore, the downregulation of HLA class I may be associated with the activation of transforming growth factor-β signaling pathway in the TME.
Conclusions: Immune escape from cytotoxic T lymphocytes may be induced in the TME in patients with unresectable advanced or recurrent G/GEJ cancer after anti-PD-1 therapy due to the downregulation of HLA class I and MHC class II expression on tumor cells. TR may be a promising treatment strategy for these patients when TR is feasible after anti-PD-1 therapy.
Keywords: HLA class I; TGF-β; anti-PD-1 therapy; gastric/gastroesophageal cancer; tumor immune microenvironment; tumor resection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Bang Y.-J., Van Cutsem E., Feyereislova A., Chung H.C., Shen L., Sawaki A., Lordick F., Ohtsu A., Omuro Y., Satoh T., et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. - DOI - PubMed
-
- Janjigian Y.Y., Shitara K., Moehler M., Garrido M., Salman P., Shen L., Wyrwicz L., Yamaguchi K., Skoczylas T., Campos Bragagnoli A., et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. doi: 10.1016/S0140-6736(21)00797-2. - DOI - PMC - PubMed
-
- Kang Y.K., Chen L.T., Ryu M.H., Oh D.Y., Oh S.C., Chung H.C., Lee K.W., Omori T., Shitara K., Sakuramoto S., et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234–247. doi: 10.1016/S1470-2045(21)00692-6. - DOI - PubMed
-
- Rha S.Y., Oh D.Y., Yanez P., Bai Y., Ryu M.H., Lee J., Rivera F., Alves G.V., Garrido M., Shiu K.K., et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1181–1195. doi: 10.1016/S1470-2045(23)00515-6. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

