Exploiting TCR Repertoire Analysis to Select Therapeutic TCRs for Cancer Immunotherapy
- PMID: 40801654
- PMCID: PMC12346228
- DOI: 10.3390/cells14151223
Exploiting TCR Repertoire Analysis to Select Therapeutic TCRs for Cancer Immunotherapy
Abstract
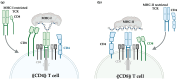
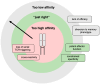
Over the past decade, numerous innovative immunotherapy strategies have transformed the treatment of cancer and improved the survival of patients unresponsive to conventional chemotherapy and radiation therapy. Immune checkpoint inhibition approaches aim to block negative regulatory pathways that limit the function of endogenous T cells, while adoptive cell therapy produces therapeutic T cells with high functionality and defined cancer specificity. While CAR engineering successfully targets cancer surface antigens, TCR engineering enables targeting of the entire cancer proteome, including mutated neo-antigens. To date, TCR engineering strategies have focused on the identification of target cancer antigens recognised by well-characterised therapeutic TCRs. In this review, we explore whether antigen-focused approaches could be complemented by TCR-focused approaches, whereby information of the TCR repertoire of individual patients provides the basis for selecting TCRs to engineer autologous T cells for adoptive cell therapy. We discuss how TCR clonality profiles, distribution in T cell subsets, and bioinformatic screening against continuously improving TCR databases can guide the selection of TCRs for therapeutic application. We further outline in vitro approaches to prioritise TCR candidates to confirm cancer reactivity and exclude recognition of healthy autologous cells, which could provide validation for their therapeutic use even when the target antigen remains unknown.
Keywords: T cell receptor; TCR repertoire; TCR sequencing; TCR-T cells; cancer immunotherapy; cell therapy; clonality; meta-clonotype; tumour infiltrating lymphocytes.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the review.
Figures


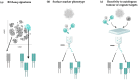
Similar articles
-
TCR-Based Therapy Directed against Kallikrein-Related Peptidase 4 Is Safe and Effective against Prostate Cancer.Cancer Immunol Res. 2025 Aug 1;13(8):1145-1159. doi: 10.1158/2326-6066.CIR-24-0119. Cancer Immunol Res. 2025. PMID: 40387827
-
Integrated system for screening tumor-specific TCRs, epitopes, and HLA subtypes using single-cell sequencing data.J Immunother Cancer. 2025 Jul 31;13(7):e012029. doi: 10.1136/jitc-2025-012029. J Immunother Cancer. 2025. PMID: 40744664 Free PMC article.
-
Metabolic reprogramming via an engineered PGC-1α improves human chimeric antigen receptor T-cell therapy against solid tumors.J Immunother Cancer. 2023 Mar;11(3):e006522. doi: 10.1136/jitc-2022-006522. J Immunother Cancer. 2023. PMID: 36914208 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Rapid parallel reconstruction and specificity screening of hundreds of T cell receptors.Nat Protoc. 2025 Mar;20(3):539-586. doi: 10.1038/s41596-024-01061-4. Epub 2024 Nov 8. Nat Protoc. 2025. PMID: 39516267 Free PMC article. Review.
References
-
- Rosenberg S.A., Packard B.S., Aebersold P.M., Solomon D., Topalian S.L., Toy S.T., Simon P., Lotze M.T., Yang J.C., Seipp C.A., et al. Use of Tumor-Infiltrating Lymphocytes and Interleukin-2 in the Immunotherapy of Patients with Metastatic Melanoma. N. Engl. J. Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. - DOI - PubMed
-
- Rosenberg S.A., Lotze M.T., Muul L.M., Leitman S., Chang A.E., Ettinghausen S.E., Matory Y.L., Skibber J.M., Shiloni E., Vetto J.T., et al. Observations on the Systemic Administration of Autologous Lymphokine-Activated Killer Cells and Recombinant Interleukin-2 to Patients with Metastatic Cancer. N. Engl. J. Med. 1985;313:1485–1492. doi: 10.1056/NEJM198512053132327. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

