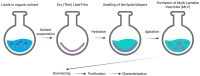
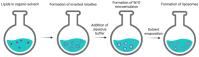
Enzyme Encapsulation in Liposomes: Recent Advancements in the Pharmaceutical and Food Sector
- PMID: 40801689
- PMCID: PMC12348261
- DOI: 10.3390/nano15151149
Enzyme Encapsulation in Liposomes: Recent Advancements in the Pharmaceutical and Food Sector
Abstract
Nanocarriers have found numerous applications in pharmaceutical and food sectors due to their unique physical and chemical properties. In particular, liposomes are the most extensively studied kind of nanoparticles for these applications. They are spherical colloidal systems characterized by lipid membranes enclosing an aqueous core. This versatile structure enables the incorporation of hydrophilic, hydrophobic, and amphiphilic molecules, making them optimal candidates for the controlled release of drugs and enzymes. Despite numerous promising applications, liposomes face challenges such as low colloidal stability, inefficient drug encapsulation, and high production costs for large-scale applications. For this reason, innovative methods, such as microfluidics, electroporation, and supercritical CO2, are currently being investigated to overcome these limitations. This review examines the recent applications of liposomes in enzyme encapsulation within the pharmaceutical and food sectors, emphasizing production challenges and emerging technological developments.
Keywords: drug administration; enzymes encapsulation; food preservation; liposomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Nucleic Acid Nanocapsules as a New Platform to Deliver Therapeutic Nucleic Acids for Gene Regulation.Acc Chem Res. 2025 Jul 1;58(13):1951-1962. doi: 10.1021/acs.accounts.5c00126. Epub 2025 Jun 9. Acc Chem Res. 2025. PMID: 40491030
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- Elabed S., Sheirf A., Ali M. Nanostructures for Cancer Therapeutics and Diagnostics: Recent Advances and Future Outlook. Radiat. Phys. Chem. 2025;226:112295. doi: 10.1016/j.radphyschem.2024.112295. - DOI
-
- Jahadi M., Khosravi-Darani K., Ehsani M.R., Pimentel T.C., Gomes da Cruz A., Mozafari M.R. Accelerating Ripening of Iranian White Brined Cheesesusing Liposome-Encapsulated and Free Proteinases. Biointerface Res. Appl. Chem. 2020;10:4966–4971. doi: 10.33263/briac101.966971. - DOI
Publication types
LinkOut - more resources
Full Text Sources