Catalytically Active Oxidized PtOx Species on SnO2 Supports Synthesized via Anion Exchange Reaction for 4-Nitrophenol Reduction
- PMID: 40801699
- PMCID: PMC12348751
- DOI: 10.3390/nano15151159
Catalytically Active Oxidized PtOx Species on SnO2 Supports Synthesized via Anion Exchange Reaction for 4-Nitrophenol Reduction
Abstract
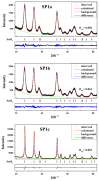
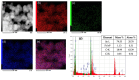
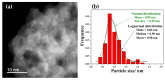
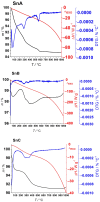
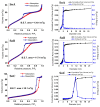
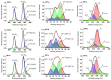
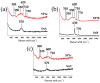
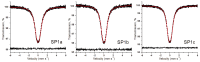
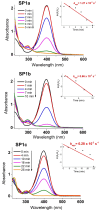
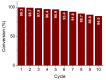
An anion exchange-assisted technique was used for the synthesis of platinum-decorated SnO2 supports, providing nanocatalysts with enhanced activity for the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP). In this study, a series of SnO2 supports, namely SnA (synthesized almost at room temperature), SnB (hydrothermally treated at 180 °C), and SnC (annealed at 600 °C), are systematically investigated, all loaded with 1 mol% Pt from H2PtCl6 under identical mild conditions. The chloride ions from the SnCl4 precursors were efficiently removed via a strong-base anion exchange reaction, resulting in highly dispersed, crystalline ~5 nm cassiterite SnO2 particles. All Pt/SnO2 composites displayed mesoporous structures with type IVa isotherms and H2-type hysteresis, with SP1a (Pt on SnA) exhibiting the largest surface area (122.6 m2/g) and the smallest pores (~3.5 nm). STEM-HAADF imaging revealed well-dispersed PtOx domains (~0.85 nm), while XPS confirmed the dominant Pt4+ and Pt2+ species, with ~25% Pt0 likely resulting from photoreduction and/or interactions with Sn-OH surface groups. Raman spectroscopy revealed three new bands (260-360 cm-1) that were clearly visible in the sample with 10 mol% Pt and were due to the vibrational modes of the PtOx species and Pt-Cl bonds introduced due the addition and hydrolysis of H2PtCl6 precursor. TGA/DSC analysis revealed the highest mass loss for SP1a (~7.3%), confirming the strong hydration of the PtOx domains. Despite the predominance of oxidized PtOx species, SP1a exhibited the highest catalytic activity (kapp = 1.27 × 10-2 s-1) and retained 84.5% activity for the reduction of 4-NP to 4-AP after 10 cycles. This chloride-free low-temperature synthesis route offers a promising and generalizable strategy for the preparation of noble metal-based nanocatalysts on oxide supports with high catalytic activity and reusability.
Keywords: 119Sn Mössbauer; 4-nitrophenol; Dowex 550; SnO2; XPS; anion exchange; catalyst; platinum.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




Similar articles
-
Microwave-Assisted Synthesis of Pt/SnO2 for the Catalytic Reduction of 4-Nitrophenol to 4-Aminophenol.Nanomaterials (Basel). 2023 Sep 2;13(17):2481. doi: 10.3390/nano13172481. Nanomaterials (Basel). 2023. PMID: 37686989 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Electrophoresis.2025 Jul 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36251838 Free Books & Documents.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Thermal stability and storage of human insulin.Cochrane Database Syst Rev. 2023 Nov 6;11(11):CD015385. doi: 10.1002/14651858.CD015385.pub2. Cochrane Database Syst Rev. 2023. PMID: 37930742 Free PMC article.
References
-
- Wacławek S., Padil V.V., Černík M. Major Advances and Challenges in Heterogeneous Catalysis for Environmental Applications: A Review. Ecol. Chem. Eng. S. 2018;25:9–34. doi: 10.1515/eces-2018-0001. - DOI
-
- Lagunova V., Filatov E., Plyusnin P., Kostin G., Urlukov A., Potemkin D., Korenev S. Metal-oxide catalysts for CO TOX and PROX processes in the Pt–Cr/Mo/W systems. Int. J. Hydrogen Energy. 2022;48:25133–25143. doi: 10.1016/j.ijhydene.2022.09.086. - DOI
-
- Okumura K., Aikawa S., Aoki Y., Abdullahi A., Mohammed M. Rational Design of Pt Supported Catalysts for Hydrosilylation: Influence of Support and Calcination Temperature. Innov. Chem. Mater. Sustain. 2025;2:74–82. doi: 10.63654/icms.2025.02074. - DOI
-
- Mukri B.D., Waghmare U.V., Hegde M.S. Platinum Ion-Doped TiO2: High Catalytic Activity of Pt2+ with Oxide Ion Vacancy in Ti4+1–xPt2+xO2–x Compared to Pt4+ without Oxide Ion Vacancy in Ti4+1–xPt4+xO2. Chem. Mater. 2013;25:3822–3833. doi: 10.1021/cm4015404. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

