Vibrational and Resistance Responses for Ether-Amine Solutions of the Buckypaper-Based Chemiresistor Sensor
- PMID: 40801735
- PMCID: PMC12348280
- DOI: 10.3390/nano15151197
Vibrational and Resistance Responses for Ether-Amine Solutions of the Buckypaper-Based Chemiresistor Sensor
Abstract
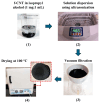
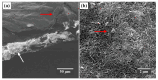
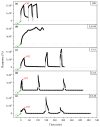
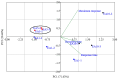
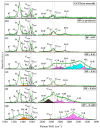
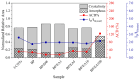
The development of miniaturized sensors has become relevant for the detection of chemical/biological substances, since they use and detect low concentrations, such as flocculants based on amines for the mining industry. In this study, buckypaper (BP) films based on carboxylic acid functionalized multi-walled carbon nanotubes (f-MWCNTs) were produced through vacuum filtration on cellulose filter paper to carry out sensory function in samples containing ether-amine (volumes: 1%, 5%, 10% and 100%). The morphological characterization of the BPs by scanning electron microscopy showed f-MWCNT aggregates randomly distributed on the cellulose fibers. Vibrational analysis by Raman spectroscopy indicated bands and sub-bands referring to f-MWCNTs and vibrational modes corresponding to chemical bonds present in the ether-amine (EA). The electrical responses of the BP to the variation in analyte concentration showed that the sensor differentiates deionized water from ether-amine, as well as the various concentrations present in the different analytes, exhibiting response time of 3.62 ± 0.99 min for the analyte containing 5 vol.% EA and recovery time of 21.16 ± 2.35 min for the analyte containing 10 vol.% EA, revealing its potential as a real-time response chemiresistive sensor.
Keywords: buckypaper; ether-amine; selectivity; sensor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Nakhaei F., Irannajad M. Reagents types in flotation of iron oxide minerals: A review. Miner. Process. Extr. Metall. Rev. 2017;39:89–124. doi: 10.1080/08827508.2017.1391245. - DOI
-
- Fan G., Wang L., Cao Y., Li C. Collecting Agent–Mineral Interactions in the Reverse Flotation of Iron Ore: A Brief Review. Minerals. 2020;10:681. doi: 10.3390/min10080681. - DOI
-
- Batisteli G.M.B., Peres A.E.C. Residual amine in iron ore flotation. Miner. Eng. 2008;21:873–876. doi: 10.1016/j.mineng.2008.04.002. - DOI
-
- Silva F.M.F. Master’s Dissertation. Federal University of Ouro Preto; Ouro Preto, Brazil: 2009. Quantification of Ether-Amines in Iron Ore Flotation Tailings as a Function of Particle Size.
-
- Nogueira S.C.S., Matos V.E., Pereira C.A., Henriques A.B., Peres A.E.C. Collector mixtures and their synergistic effect on quartz floatability. Int. Eng. J. 2022;75:371–378. doi: 10.1590/0370-44672022750002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

