CD81+ senescent-like fibroblasts exaggerate inflammation and activate neutrophils via C3/C3aR1 axis in periodontitis
- PMID: 40801798
- PMCID: PMC12349900
- DOI: 10.7554/eLife.96908
CD81+ senescent-like fibroblasts exaggerate inflammation and activate neutrophils via C3/C3aR1 axis in periodontitis
Abstract
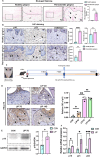
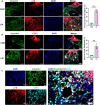
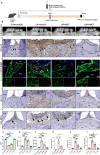
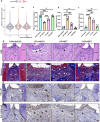
Periodontitis, a prevalent inflammatory disease worldwide, poses a significant economic burden on society and the country. Previous research has established a connection between cellular senescence and periodontitis. However, the role and mechanism of cell senescence in the progression of periodontitis have not been thoroughly investigated. This study aimed to explore the involvement of cellular senescence in the pathogenesis of periodontitis and determine the underlying mechanisms. Our findings demonstrated that senescent cells accumulated during the progress of periodontitis in both human samples and mice models. Moreover, several scRNA-seq analyses suggested that gingival fibroblasts were the main cell population undergoing cellular senescence during human periodontitis, which helps mitigate tissue damage and bone loss. Furthermore, we identified a high expression of CD81 in the senescent gingival fibroblast population. These cells were found to actively contribute to inflammation through their potent pro-inflammatory metabolic activity and secretion of senescence-associated secretory phenotype factors. Additionally, they recruited neutrophils via the C3/C3aR1 pathway, indirectly sustaining the inflammatory response. Senolytics via Navitoclax successfully alleviated inflammation and bone loss in periodontitis, and administration of metformin could alleviate inflammation and bone loss in periodontitis through inhibiting cellular senescence. These results provide valuable insights into the cellular and molecular basis of periodontitis-induced tissue damage, highlighting the significance of fibroblast senescence. In conclusion, our study sheds light on the relationship between CD81 and cellular senescence, suggesting its potential as a therapeutic target for periodontitis.
Keywords: CD81; cellular senescence; human; human gingival fibroblasts; immunology; inflammation; mouse; periodontal diseases; senescence-associated secretory phenotype.
© 2024, Fu, Yin et al.
Conflict of interest statement
LF, CY, QZ, SG, WS, TX, QS, LC, JL, MW, HX No competing interests declared
Figures














Update of
- doi: 10.1101/2024.02.21.581332
- doi: 10.7554/eLife.96908.1
- doi: 10.7554/eLife.96908.2
References
-
- Alayash Z, Baumeister S-E, Holtfreter B, Kocher T, Baurecht H, Ehmke B, Nolde M, Reckelkamm SL. Complement C3 as a potential drug target in periodontitis: evidence from the cis-Mendelian randomization approach. Journal of Clinical Periodontology. 2024;51:127–134. doi: 10.1111/jcpe.13894. - DOI - PubMed
-
- Ando Y, Tsukasaki M, Huynh NC-N, Zang S, Yan M, Muro R, Nakamura K, Komagamine M, Komatsu N, Okamoto K, Nakano K, Okamura T, Yamaguchi A, Ishihara K, Takayanagi H. The neutrophil-osteogenic cell axis promotes bone destruction in periodontitis. International Journal of Oral Science. 2024;16:18. doi: 10.1038/s41368-023-00275-8. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

