Mapping Excited-State Decay Mechanisms in Acetylacetone by Sub-20 fs Time-Resolved Photoelectron Spectroscopy
- PMID: 40801839
- PMCID: PMC12395474
- DOI: 10.1021/jacs.5c06327
Mapping Excited-State Decay Mechanisms in Acetylacetone by Sub-20 fs Time-Resolved Photoelectron Spectroscopy
Abstract
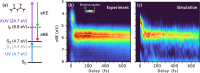
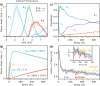
Excited-State Intramolecular Hydrogen Transfer (ESIHT) is one of the fastest chemical reactions, occurring on the order of tens of femtoseconds and playing a critical role in light-driven biological processes and technological applications. Here, we investigate the early stages of coupled nuclear-electron dynamics using acetylacetone (AcAc) as a model system exhibiting ESIHT. We employ ultraviolet-extreme ultraviolet (UV-XUV) time-resolved photoelectron spectroscopy (tr-PES) with sub-20 fs resolution in combination with high-level dynamically correlated simulations (CASPT2) to map the electronic relaxation pathways and vibrational modes driving this process. Our results provide distinct spectroscopic signatures of ESIHT occurring within the first 20 fs and resolve the active vibrational modes, showing the intricate evolution of the electronic and nuclear degrees of freedom. Moreover, the analysis reveals the key role of ultrafast intersystem crossing (ISC) to triplet states in modulating the excited-state dynamics and its implications for the overall relaxation pathways. These findings refine our understanding of the photochemistry of AcAc and suggest general principles that can be applied to similar conjugated systems.
Figures


Similar articles
-
Host-Guest Charge-Transfer Mediated Photoredox Catalysis Inside Water-Soluble Nanocages.Acc Chem Res. 2025 Aug 19;58(16):2600-2612. doi: 10.1021/acs.accounts.5c00342. Epub 2025 Jul 31. Acc Chem Res. 2025. PMID: 40742211
-
Ultrafast solvent migration in an iron complex revealed by nonadiabatic dynamics simulations.Chem Sci. 2025 May 13;16(24):11128-11137. doi: 10.1039/d5sc01174d. eCollection 2025 Jun 18. Chem Sci. 2025. PMID: 40417306 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Gender differences in the context of interventions for improving health literacy in migrants: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2024 Dec 12;12(12):CD013302. doi: 10.1002/14651858.CD013302.pub2. Cochrane Database Syst Rev. 2024. PMID: 39665382
References
-
- Wang, L. ; Xiao, J. . Hydrogen-atom transfer reactions. Hydrogen Transfer Reactions; Guillena, G. , Ramón, D. , Eds.; Springer: New York, 2016; pp 205–259.
-
- Douhal A., Lahmani F., Zewail A. H.. Proton-transfer reaction dynamics. Chem. Phys. 1996;207:477–498. doi: 10.1016/0301-0104(96)00067-5. - DOI
-
- Formosinho S. J., Arnaut L. G.. Excited-state proton transfer reactions II. intramolecular reactions. J. Photochem. Photobiol., A. 1993;75:21–48. doi: 10.1016/1010-6030(93)80158-6. - DOI
-
- Barbatti M., Aquino A. J., Lischka H., Schriever C., Lochbrunner S., Riedle E.. Ultrafast internal conversion pathway and mechanism in 2-(2-´hydroxyphenyl) benzothiazole: a case study for excited-state intramolecular proton transfer systems. Phys. Chem. Chem. Phys. 2009;11(9):1406–1415. doi: 10.1039/b814255f. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

