JAK2/STAT3 Signaling Pathway Modulates Acute Methylmercury Toxicity in the Mouse Astrocyte C8-D1A Cell Line
- PMID: 40802001
- PMCID: PMC12350482
- DOI: 10.1007/s11064-025-04507-7
JAK2/STAT3 Signaling Pathway Modulates Acute Methylmercury Toxicity in the Mouse Astrocyte C8-D1A Cell Line
Abstract
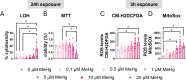
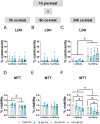
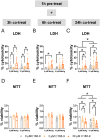
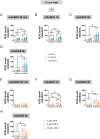
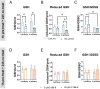
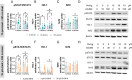
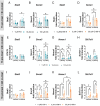
Methylmercury (MeHg), an environmental pollutant, reaches the human body predominantly through contaminated fish consumption, potentially leading to severe neurological disorders. Upon ingestion MeHg reaches the brain and selectively accumulates in astrocytes. The activation of nuclear factor erythroid 2-related factor 2 (Nrf2) has been identified as a key early response to MeHg-induced oxidative injury, positioning it as a potential therapeutic target. However, recent studies suggest that Nrf2 activation alone may not be sufficient to mitigate MeHg toxicity, indicating the existence of other protective mechanisms. The signal transducer and activator of transcription 3 (STAT3) signaling pathway, known for its role in cell growth and survival, has emerged as a potential player in redox homeostasis. In this study, we investigated the role of STAT3 in acute (≤ 24 h) MeHg-induced neurotoxicity. MeHg exposure induced STAT3 expression in C8-D1A astrocytic cells. Our data demonstrated that pharmacological inhibition of STAT3 using AG490 or C188-9 exacerbated MeHg-induced cell death and compromised antioxidant responses. Furthermore, to fully characterize the role of STAT3 in oxidative stress, we used two different antioxidants, N-acetylcysteine (NAC) and Trolox. Conversely, reactive oxygen species (ROS)-scavenging antioxidants partially ameliorated STAT3 activation, suggesting that MeHg-induced STAT3 activation is mediated, at least in part, by mechanisms independent of ROS. Our findings suggest that STAT3 contributes to neuroprotection against MeHg exposure in astrocytes and is, at least in part, regulated by the increase in ROS levels within these cells.
Keywords: Astrocytes; Inflammation; Methylmercury; Neurotoxicity; Oxidative stress.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing Interests: The authors declare no competing interests.
Figures





Update of
-
JAK2/STAT3 signaling pathway mediates methylmercury toxicity in mouse astrocyte neuronal C8-D1A cell line.bioRxiv [Preprint]. 2024 Oct 25:2024.07.13.603400. doi: 10.1101/2024.07.13.603400. bioRxiv. 2024. Update in: Neurochem Res. 2025 Aug 13;50(4):265. doi: 10.1007/s11064-025-04507-7. PMID: 39071366 Free PMC article. Updated. Preprint.
Similar articles
-
JAK2/STAT3 signaling pathway mediates methylmercury toxicity in mouse astrocyte neuronal C8-D1A cell line.bioRxiv [Preprint]. 2024 Oct 25:2024.07.13.603400. doi: 10.1101/2024.07.13.603400. bioRxiv. 2024. Update in: Neurochem Res. 2025 Aug 13;50(4):265. doi: 10.1007/s11064-025-04507-7. PMID: 39071366 Free PMC article. Updated. Preprint.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Methylmercury induces inflammatory response and autophagy in microglia through the activation of NLRP3 inflammasome.Environ Int. 2024 Apr;186:108631. doi: 10.1016/j.envint.2024.108631. Epub 2024 Apr 4. Environ Int. 2024. PMID: 38588609
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Antioxidant treatments for schizophrenia.Cochrane Database Syst Rev. 2016 Feb 5;2(2):CD008919. doi: 10.1002/14651858.CD008919.pub2. Cochrane Database Syst Rev. 2016. PMID: 26848926 Free PMC article.
References
-
- W. World Health Organization, Mercury and health, (2017)
-
- Depew DC, Burgess NM, Campbell LM (2013) Spatial patterns of Methylmercury risks to common loons and piscivorous fish in Canada. Environ Sci Technol 47:13093–13103 - PubMed
-
- Wren CD (1986) A review of metal accumulation and toxicity in wild mammals. I. Mercury. Environ Res 40:210–244 - PubMed
-
- E. Environmental Protection Agency, Basic Information about Mercury, (2023)
-
- Cariccio VL, Sama A, Bramanti P, Mazzon E (2019) Mercury involvement in neuronal damage and in neurodegenerative diseases. Biol Trace Elem Res 187:341–356 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

