Combined experimental and computational investigation of vildagliptin: spectroscopy, electronic structure, MD and Docking to EGFR, VEGFR2, and HER2 anticancer targets
- PMID: 40802014
- PMCID: PMC12350465
- DOI: 10.1007/s10822-025-00646-9
Combined experimental and computational investigation of vildagliptin: spectroscopy, electronic structure, MD and Docking to EGFR, VEGFR2, and HER2 anticancer targets
Abstract
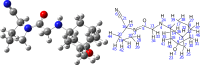
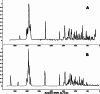
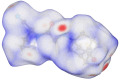
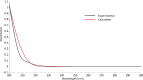
This study combines experimental and computational approaches to investigate the molecular geometry and physicochemical properties of vildagliptin (VILD). Using methods such as UV-Vis, spectrofluorimetry, FTIR/Raman, and circular dichroism alongside DFT, molecular docking, and dynamics simulations, a reliable molecular model was obtained that aligns closely with X-ray crystallographic data. This model enabled accurate predictions of vibrational frequencies and systematic assignments of vibrational modes. Analyses, including Hirshfeld surface mapping, molecular electrostatic potential, HOMO-LUMO energetics, Fukui indices, and natural population analysis, provided clear insights into VILD's reactivity, while NBO and TD-DFT studies elucidated key stabilizing interactions and high-energy electronic transitions. NTO visualization further clarified orbital dynamics, and circular dichroism measurements explained the molecular basis of the Cotton effect. Additionally, molecular docking and molecular dynamics simulations confirmed the formation of stable complexes with EGFR, VEGFR2, and HER2 receptor proteins, suggesting potential anticancer activity. The main purpose of this publication is to fill existing gaps in our understanding of VILD's molecular behavior and offer a robust foundation for rational drug design and improved therapeutic strategies.
Keywords: DFT; FTIR; Raman; Spectroscopy; Vildagliptin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The author declare no competing interests.
Figures











Similar articles
-
Quantum chemical modeling, molecular docking, and ADMET evaluation of imidazole phenothiazine hybrids.Sci Rep. 2025 Jul 2;15(1):23413. doi: 10.1038/s41598-025-90495-1. Sci Rep. 2025. PMID: 40604198 Free PMC article.
-
Quantum DFT analysis and molecular docking investigation of various potential breast cancer drugs.J Mater Chem B. 2024 Dec 18;13(1):218-238. doi: 10.1039/d4tb01803f. J Mater Chem B. 2024. PMID: 39545283
-
Exploitation of novel pyrazolo[3,4-d]pyrimidine scaffold tethered to thiazole as potential EGFR/HER2 dual kinase inhibitor to overcome lapatinib resistant breast cancer: Design, synthesis, in silico docking and molecular dynamic simulation.Bioorg Chem. 2025 Aug;163:108671. doi: 10.1016/j.bioorg.2025.108671. Epub 2025 Jun 3. Bioorg Chem. 2025. PMID: 40480106
-
Comparative Linkage of Novel Anti-Tumor Pd(II) Complex with Bio-Macromulecules: Fluorescence, UV-Vis, DFT, Molecular Docking and Molecular Dynamics Simulation Studies.J Fluoresc. 2025 Jun;35(6):4255-4276. doi: 10.1007/s10895-024-03820-8. Epub 2024 Jul 5. J Fluoresc. 2025. PMID: 38967860
-
Traditional Chinese medicinal herbs combined with epidermal growth factor receptor tyrosine kinase inhibitor for advanced non-small cell lung cancer: a systematic review and meta-analysis.J Integr Med. 2014 Jul;12(4):346-58. doi: 10.1016/S2095-4964(14)60034-0. J Integr Med. 2014. PMID: 25074884
References
-
- Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH (2021) SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet 398:262–276. 10.1016/S0140-6736(21)00536-5 - PubMed
-
- Knapen LM, de Jong RGPJ, Driessen JHM, Keulemans YC, van Erp NP, De Bruin ML, Leufkens HGM, Croes S, de Vries F (2017) Use of incretin agents and risk of acute and chronic pancreatitis: A population-based cohort study. Diabetes Obes Metab 19:401–411. 10.1111/dom.12833 - PubMed
-
- Egan AG, Blind E, Dunder K, de Graeff PA, Hummer BT, Bourcier T, Rosebraugh C (2014) Pancreatic safety of Incretin-Based Drugs — FDA and EMA assessment. N Engl J Med 370:794–797. 10.1056/NEJMp1314078 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

