Targeting CDK2 for cancer therapy
- PMID: 40802510
- PMCID: PMC12477701
- DOI: 10.1016/j.celrep.2025.116140
Targeting CDK2 for cancer therapy
Abstract
Targeting cell-cycle regulatory processes by inhibiting cyclin-dependent kinases (CDKs) has long been considered a significant therapeutic strategy for oncology. Recent studies have highlighted the complexity of targeting CDK2 for cancer therapy. Unlike CDK4/6 inhibitors, CDK2 inhibitors can impact different phases of the cell cycle by modulating distinct effector pathways, and the response to CDK2 inhibitors is controlled by the genetic and epigenetic makeup of the tumor. Biomarkers have emerged that can inform the effective use of these drugs and include cyclin E and p16INK4A. Work across several different tumor types indicates that CDK2 inhibitors can be combined effectively with various drug classes. However, more investigation is needed to understand the potential limitations and drug toxicities of existing CDK2 inhibitors and those in development.
Keywords: CDK inhibitors; CDK2; CDK4/6; CDKN2A; CP: Cancer; RB; cell cycle; cyclin D1; cyclin E; p16IN4A.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests I.S. received research funding from Novartis. E.S.K. received research funding from Blueprint Medicines, Bristol Myers Squibb, and Aleksia Therapeutics and consults through Cancer Cell Cycles-LLC. A.K.W. received research funding from Blueprint Medicines, Bristol Myers Squibb, and Aleksia Therapeutics.
Figures
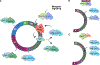

References
-
- Sherr CJ (1996). Cancer cell cycles. Science 274, 1672–1677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

