Passage of human-origin influenza A virus in swine tracheal epithelial cells selects for adaptive mutations in the hemagglutinin gene
- PMID: 40802672
- PMCID: PMC12349064
- DOI: 10.1371/journal.pone.0327096
Passage of human-origin influenza A virus in swine tracheal epithelial cells selects for adaptive mutations in the hemagglutinin gene
Abstract
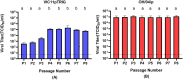
Frequent spillover of influenza A viruses from humans to swine contributes to the increasing diversity of influenza viruses circulating in pigs. Although these events are common, little is known about the adaptation processes that take place when viruses jump between the two species. We examined the changes that occurred during serial passages of a reassortant H3N2 virus (VIC11pTRIG) containing human seasonal surface genes (Hemagglutinin and Neuraminidase) and a swine-adapted internal gene constellation in differentiated primary swine tracheal epithelial cells (pSTECs). The VIC11pTRIG reassortant virus was serially passaged 8 times in pSTECs and compared to a control swine-adapted strain (OH/04p) containing the same internal gene constellation. Viral RNA from passages 0 (inoculum), 1, 3, 4-8 were sequenced via next generation or Sanger sequencing. Hemagglutinin diversity was highest at passage 3. Two amino acid mutations in the Hemagglutinin protein (N165K and N216K) were fixed at passages 7 and 5, respectively. These changes were associated with increased fitness of the virus in pSTECs compared to the original parental strain. Our results suggest that the adaptation of human seasonal H3N2 to swine cells may lead to the selection of HA mutations located near the receptor binding site. These mutations may result in increased fitness of human-origin H3N2 strains to adapt in swine.
Copyright: © 2025 Mo et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
2018-2019 human seasonal H3N2 influenza A virus spillovers into swine with demonstrated virus transmission in pigs were not sustained in the pig population.J Virol. 2024 Dec 17;98(12):e0008724. doi: 10.1128/jvi.00087-24. Epub 2024 Nov 11. J Virol. 2024. PMID: 39526773 Free PMC article.
-
Amino acid 138 in the HA of a H3N2 subtype influenza A virus increases affinity for the lower respiratory tract and alveolar macrophages in pigs.PLoS Pathog. 2024 Feb 20;20(2):e1012026. doi: 10.1371/journal.ppat.1012026. eCollection 2024 Feb. PLoS Pathog. 2024. PMID: 38377132 Free PMC article.
-
Antigenic Characterization and Pandemic Risk Assessment of North American H1 Influenza A Viruses Circulating in Swine.Microbiol Spectr. 2022 Dec 21;10(6):e0178122. doi: 10.1128/spectrum.01781-22. Epub 2022 Nov 1. Microbiol Spectr. 2022. PMID: 36318009 Free PMC article.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
-
Surgical interventions for treating intracapsular hip fractures in older adults: a network meta-analysis.Cochrane Database Syst Rev. 2022 Feb 14;2(2):CD013404. doi: 10.1002/14651858.CD013404.pub2. Cochrane Database Syst Rev. 2022. PMID: 35156192 Free PMC article.
References
LinkOut - more resources
Full Text Sources

