Lipid nanoparticles for mRNA delivery in brain via systemic administration
- PMID: 40802746
- PMCID: PMC12346281
- DOI: 10.1126/sciadv.adw0730
Lipid nanoparticles for mRNA delivery in brain via systemic administration
Abstract
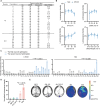
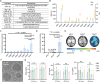
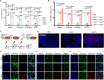
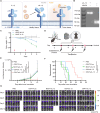
Efficient delivery of messenger RNA (mRNA) to the brain via systemic administration remains a challenge, primarily due to the blood-brain barrier. To address this challenge, we incorporated SR-57227, a ligand of serotonin [5-hydroxytryptamine type 3 (5-HT3)] receptor, into the design of ionizable lipids to develop lipid nanoparticles (LNPs) for systemic mRNA delivery to the brain. OS4T LNP was identified as an optimized formulation based on multiple assays. Following systemic administration, OS4T LNP achieved over a 50-fold increase in mRNA translation within brain tissues compared to US Food and Drug Administration-approved Onpattro LNPs (DLin-MC3-DMA). In an orthotopic glioblastoma (GBM) mouse model, engineered interleukin-12 mRNA-loaded OS4T LNPs significantly suppressed tumor growth and improved overall survival. This study demonstrates OS4T LNP as a promising platform for brain mRNA delivery and highlights its potential for treating GBM and other central nervous system disorders.
Figures

References
-
- Barbier A. J., Jiang A. Y., Zhang P., Wooster R., Anderson D. G., The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 40, 840–854 (2022). - PubMed
-
- Hajj K. A., Whitehead K. A., Tools for translation: Non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2, 17056 (2017).
-
- Loughrey D., Dahlman J. E., Non-liver mRNA delivery. Acc. Chem. Res. 55, 13–23 (2022). - PubMed
-
- Sun B., Wu W., Narasipura E. A., Ma Y., Yu C., Fenton O. S., Song H., Engineering nanoparticle toolkits for mRNA delivery. Adv. Drug Deliv. Rev. 200, 115042 (2023). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

