Src/Fas2-dependent Ephrin phosphorylation initiates Eph/Ephrin reverse signaling through Rac1 to shape columnar units in the fly brain
- PMID: 40802768
- PMCID: PMC12346307
- DOI: 10.1126/sciadv.adv7490
Src/Fas2-dependent Ephrin phosphorylation initiates Eph/Ephrin reverse signaling through Rac1 to shape columnar units in the fly brain
Abstract
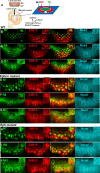
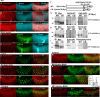
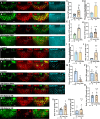
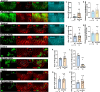
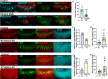
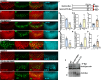
Columns are the morphological and functional units containing multiple neurons in the brain. The molecular mechanisms of column formation are largely unknown. Ephrin/Eph signaling mediates a variety of developmental processes. Ephrin acts as a ligand for Eph to regulate forward signaling, whereas Eph acts as a ligand for Ephrin to regulate reverse signaling. However, whether and how the uni- or bidirectional Ephrin/Eph signaling is involved in column formation remains elusive. In this study, we show that Ephrin and Eph regulate the morphology and location of columnar neurons through bidirectional repulsive signaling. Furthermore, Eph ligand triggers cytoplasmic tyrosine phosphorylation of Ephrin under the control of Src kinases and Fasciclin 2 (Fas2), forming the Ephrin/Src/Fas2 complex to promote reverse signaling through a downstream regulator, Rac1. This study provides the detailed analysis of the molecular interactions involved in column formation using the fly brain as a model.
Figures

References
-
- Hasegawa E., Kitada Y., Kaido M., Takayama R., Awasaki T., Tabata T., Sato M., Concentric zones, cell migration and neuronal circuits in the Drosophila visual center. Development 138, 983–993 (2011). - PubMed
-
- Fischbach K. F., Dittrich A. P. M., The optic lobe of Drosophila melanogaster. I. A Golgi analysis of wild-type structure. Cell Tissue Res. 258, 441–475 (1989).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

