Transcriptome fingerprinting of aberrant fibroblast activation unlocks effective therapeutics to tackle cardiac fibrosis
- PMID: 40802773
- PMCID: PMC12346348
- DOI: 10.1126/sciadv.adx0968
Transcriptome fingerprinting of aberrant fibroblast activation unlocks effective therapeutics to tackle cardiac fibrosis
Abstract
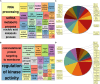
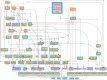
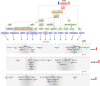
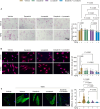
Aberrant activation of fibroblasts is a pivotal component of cardiac fibrosis predisposing to heart failure. However, the molecular regulation of the functional state of cardiac fibroblasts in fibrosis resolution remains largely unexplored, and therefore, effective antifibrosis therapies are still lacking. By translating mouse transcriptomics to humans, we unlocked common molecular denominators connecting the fibroblast phenotypic state and fibrogenic signaling pathways in cardiac fibrosis. Through the construction of a fibroblast-specific transcriptional gene regulatory network, we found ITGAL and DUSP9 as key druggable targets for human myocardial fibrosis. A computational drug repurposing approach predicted 367 antifibrotic candidate compounds for heart disease. In primary cardiac fibroblasts derived from patients with heart failure, we provided experimental validation of the top 2-ranked repositioned drug candidates and their combination. These innovative approaches facilitate the identification of potential targets and drug candidates for cardiac fibrosis, providing actionable opportunities for clinical translation.
Figures



References
-
- Aoki T., Fukumoto Y., Sugimura K., Oikawa M., Satoh K., Nakano M., Nakayama M., Shimokawa H., Prognostic impact of myocardial interstitial fibrosis in non-ischemic heart failure—Comparison between preserved and reduced ejection fraction heart failure. Circ. J. 75, 2605–2613 (2011). - PubMed
-
- Assomull R. G., Prasad S. K., Lyne J., Smith G., Burman E. D., Khan M., Sheppard M. N., Poole-Wilson P. A., Pennell D. J., Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J. Am. Coll. Cardiol. 48, 1977–1985 (2006). - PubMed
-
- Gulati A., Jabbour A., Ismail T. F., Guha K., Khwaja J., Raza S., Morarji K., Brown T. D. H., Ismail N. A., Dweck M. R., Di Pietro E., Roughton M., Wage R., Daryani Y., O’Hanlon R., Sheppard M. N., Alpendurada F., Lyon A. R., Cook S. A., Cowie M. R., Assomull R. G., Pennell D. J., Prasad S. K., Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 309, 896–908 (2013). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

