Safety of antidepressants commonly used in 6-17-year-old children and adolescents: A disproportionality analysis from 2014-2023 on the basis of the FAERS database
- PMID: 40802817
- PMCID: PMC12349705
- DOI: 10.1371/journal.pone.0330025
Safety of antidepressants commonly used in 6-17-year-old children and adolescents: A disproportionality analysis from 2014-2023 on the basis of the FAERS database
Abstract
Background: Depression is a common mental disorder in children and adolescents, and antidepressants are widely used for treatment. This study aimed to explore and analyze adverse events (AEs) associated with antidepressant use in this population, providing insights for safety assessment.
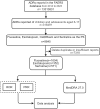
Methods: This study extracted AE reports from the US FDA Adverse Event Reporting System (FAERS) for children and adolescents aged 6-17 years from 2014-2023, with fluoxetine, escitalopram, and sertraline as the primary suspected drugs. The study used the International Dictionary of Medical Terminology (MedDRA) to encode and classify AEs, and employed the reporting odds ratio (ROR) and proportional reporting ratio (PRR) methods for data mining.
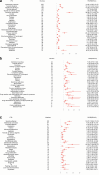
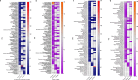
Results: The FAERS database included 9,845 AE reports involving fluoxetine, escitalopram, and sertraline among children and adolescents aged 6-17 years. After removing duplicates, the analysis yielded 1,604, 352, and 571 AEs for fluoxetine, escitalopram, and sertraline, respectively. The study population demonstrated a sex distribution with approximately twice as many female patients as male patients, with most patients aged 12-17 years. At the system organ class (SOC) level, the AEs predominantly involved psychiatric disorders and nervous system disorders. Within the psychiatric disorders category at the high level group term (HLGT) level, the most frequently reported AE signals across all three medications were suicidal and self-injurious behaviors (not elsewhere classified, hereinafter referred to as NEC) and anxiety disorders and symptoms; For nervous system disorders, the predominant AE signals were neurological disorders (NEC) and movement disorders (including parkinsonism). At the Preferred Term (PT) level, the three antidepressants demonstrated similar AEs, including various suicidal and self-injurious behaviors, intentional overdose, neurological disorders (including serotonin syndrome, extravertebral reactions, etc) and prolonged QT. The typical AEs of fluoxetine included decreased appetite, insomnia, and urinary retention. Escitalopram was associated with additional AEs of sexual dysfunction and toxic epidermal necrolysis, whereas sertraline demonstrated unique associations with headache and rhabdomyolysis.
Conclusion: This study may offer further evidence regarding AEs associated with commonly prescribed antidepressants in children and adolescents. Overall, the findings are mostly consistent with the AEs recorded in the packaging instructions and previous reports. However, the study also identified previously unreported AEs and highlighted variations in the AE profiles across different demographic groups. As the causal relationships between these medications and the observed AEs remain to be fully elucidated, additional research is warranted to confirm these findings.
Copyright: © 2025 Zhou et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist. This research was conducted independently, and no financial or personal relationships influenced the design, execution, or interpretation of the findings. The study utilized publicly available data from the FDA Adverse Event Reporting System (FAERS) database, and no external funding or support was received from pharmaceutical companies or other entities with a vested interest in the outcomes. All authors have contributed to the manuscript in an impartial manner, and the results and conclusions presented are based solely on the analysis of the data. The authors affirm their commitment to transparency and scientific integrity in reporting the findings of this study. I have read the journal's policy and the authors of this manuscript have no competing interests.
Figures

Similar articles
-
New generation antidepressants for depression in children and adolescents: a network meta-analysis.Cochrane Database Syst Rev. 2021 May 24;5(5):CD013674. doi: 10.1002/14651858.CD013674.pub2. Cochrane Database Syst Rev. 2021. PMID: 34029378 Free PMC article.
-
A pharmacovigilance study of vortioxetine based on data from the FDA adverse event reporting system.Sci Rep. 2025 Aug 7;15(1):28886. doi: 10.1038/s41598-025-13786-7. Sci Rep. 2025. PMID: 40775011 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Pharmacological intervention for irritability, aggression, and self-injury in autism spectrum disorder (ASD).Cochrane Database Syst Rev. 2023 Oct 9;10(10):CD011769. doi: 10.1002/14651858.CD011769.pub2. Cochrane Database Syst Rev. 2023. PMID: 37811711 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
References
-
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

