Infection with Mycobacterium tuberculosis alters the antibody response to HIV-1
- PMID: 40802847
- PMCID: PMC12370194
- DOI: 10.1371/journal.ppat.1013350
Infection with Mycobacterium tuberculosis alters the antibody response to HIV-1
Abstract
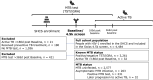
Background: Co-infection with Mycobacterium tuberculosis (MTB) differentially modulates untreated HIV-1 infection, with asymptomatic MTB reducing HIV-1 viremia and opportunistic infections and active tuberculosis (TB) accelerating AIDS progression. Here, we investigate antibody (Ab) responses to HIV-1 in people with HIV (PWH) without MTB, with asymptomatic MTB, and with later progression to active TB to elucidate MTB-associated effects on HIV-1 immune control.
Methods: Using the Swiss HIV Cohort Study (SHCS), we conducted a retrospective study that included 2,840 PWH with data on MTB status and HIV-1-specific plasma binding-/neutralizing-responses. We evaluated associations between MTB status and binding-/neutralizing-responses while adjusting for key disease and demographic parameters.
Results: Among the included 2,840 PWH, 263 PWH had asymptomatic MTB based on either a positive TST-/IGRA-test at the baseline (time of HIV-1 Ab measurement) or on later progression to active TB. Compared to PWH without MTB infection, PWH with asymptomatic MTB infection showed reduced HIV-1 Ab levels, both for Env binding (e.g., IgG1 BG505 trimer antigen, p = 0.024) and neutralization of a diverse panel of HIV-1 viruses (p = 0.012). Conversely, PWH (n = 32) who later progressed to active TB (>180 days after baseline) demonstrated a significant shift towards IgG3 in their HIV-1 Ab repertoire (p = 0.011), detectable in median 3.8 years (IQR 2.4 - 8.7) before active TB onset.
Conclusion: Our data indicate that asymptomatic MTB infection and active TB exert profound heterologous effects on HIV-1 specific Ab development. These findings advance our understanding of host-pathogen dynamics and may have implications for new diagnostic approaches in predicting future active TB.
Copyright: © 2025 Zeeb et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: K.K. has received research grants unrelated to this work from the Swiss National Science Foundation. I.A.A. received research grants from the Swiss HIV Cohort Study, Gilead and travel expenses from Gilead for research unrelated to this work. J.No. received research grants unrelated to this work from the Swiss HIV Cohort Study and the cantonal hospital St. Gallen, paid to her institution, and travel expenses from Gilead unrelated to this work. M.C. received research grants unrelated to this work from Gilead, ViiV and MSD, paid to his institution; payment for expert testimony unrelated to his work from Gilead, ViiV and MSD, paid to his institution; and travel expenses unrelated to this work from Gilead, paid to his institution. A.E. received honoraria for presentations unrelated to this work from Bavarian Nordic, paid to her institution; and honoraria for advisory board consultations unrelated to this work from ViiV and Gilead, paid to her institution. E.B. received research grants unrelated to this work from MSD, paid to his institution; consulting fees unrelated to this work from Moderna, paid to his institution; honoraria for presentations unrelated to this work from Pfizer, paid to his institution; travel expenses unrelated to this work from ViiV, MSD, Gilead and Pfizer, paid to his institution; and honoraria for data safety monitoring board or advisory board consultations unrelated to this work from ViiV, MSD, Pfizer, Gilead, Moderna, AstraZeneca, AbbVie and Ely Lilly, paid to his institution. H.F.G. has received research grants unrelated to this work from the Swiss National Science Foundation, Swiss HIV Cohort Study, Yvonne Jacob Foundation, NIH, Gilead, ViiV and Bill and Melinda Gates foundation, paid to his institution; personal honoraria for data safety monitoring board or advisory board consultations unrelated to this work from Merck, ViiV healthcare, Gilead Sciences, Janssen, Johnson and Johnson, Novartis and GSK. R.D.K. received research grants unrelated to this work from Gilead and NIH, paid to his institution. All other authors have declared that no competing interests exist.
Figures


References
-
- WHO TB Factsheet. Global Tuberculosis Report 2022 Factsheet [Internet]. 2022 [cited 2024 Jan 11]. Available from: https://www.who.int/publications/m/item/global-tuberculosis-report-2022-...
-
- Meintjes G, Maartens G. HIV-Associated Tuberculosis. New Eng J Med. 2024;391(4):343–55. - PubMed
-
- Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, et al. Tuberculosis. Nat Rev Dis Primers. 2016;2(1):16076. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

