A comparison of spermatogenesis between flies and men-conserved processes of male gamete production
- PMID: 40802929
- PMCID: PMC12584897
- DOI: 10.1093/humupd/dmaf018
A comparison of spermatogenesis between flies and men-conserved processes of male gamete production
Abstract
Background: Spermatogenesis is a dynamic process that involves the co-ordinated development of millions of cells, from stem cells to highly polarized sperm capable of motility and fertility. It is, therefore, not surprising that many thousand genes are required for male fertility. Mutant mouse models are routinely employed to test the function of these genes as well as to validate genetic variants that may be causing human male infertility. The use of mice and other animal models has led to significant knowledge gain regarding the genetic regulation of mammalian male fertility. However, due to the sheer number of genes and genetic variants to be tested these approaches are expensive and time-consuming. We and others have investigated the use of alternate model organisms to expedite validation approaches, including the utility of the fruit fly Drosophila melanogaster.
Objective and rationale: This review explores the conserved mechanisms of sperm production between mammals and flies, with a focus on the human setting where possible.
Search methods: Studies were identified via PubMed using searches including keywords related to the focus of this review, including human, mammalian, and fly or Drosophila spermatogenesis and male fertility. Follow-up searches including using search terms for specific structures and processes for comparison between species included, but were not limited to, male reproductive tract, spermatogenesis, spermatogonia and stem cell niche, meiosis, spermiogenesis and its sub-processes, and sperm/spermatozoa. No time frame or species restrictions were placed on searches.
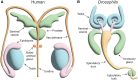
Outcomes: We identify key phases of spermatogenesis that are highly conserved between humans and flies, including the early germ cell divisions and the ratio of haploid germ cells generated for each spermatogonial stem cell, allowing their use as a model organism to explore such processes. Some processes are moderately well conserved between mammals and flies, including meiosis with the notable absence of 'crossing over' in flies. We also identify some processes that are poorly conserved, such as a divergence in sperm tail accessory structures, for which flies are not likely a suitable model organism to decipher human biology or for mammals broadly. Examples of where the fly has been or could be useful to study mammalian gene function in male fertility have also been described.
Wider implications: Drosophila melanogaster is undoubtedly a useful model organism for studying a wide range of human diseases with genetic origins, including male infertility. Both humans and flies possess a pair of testes with the primary role of generating sperm. The formation of cysts in Drosophila testes allows germ cells to constantly proliferate and stay synchronized at the respective maturation phase, as is the case for humans. While both organisms use a method of sperm storage, mammalian sperm undergo post-testicular modifications and are stored in the epididymis. In Drosophila, sperm are stored in the seminal vesicle, and do not appear to undergo any overt post-testicular modifications in this epididymis-like structure. The seminal vesicle is a separate organ in mammals that is responsible for generation of the seminal fluid. It is important to note that male fertility and thus spermatogenesis are subject to significant evolutionary pressure, and there is a degree of variation in its processes between all species. As such, the absence of a phenotype in mutants would not determine that the gene is dispensable for fertility in humans. While flies are useful for genetic studies to confirm human disease causality, we propose they should be used primarily to pre-screen and select strong candidates for further interrogation in mammalian species for translational pathways in the context of human fertility.
Registration number: N/A.
Keywords: animal model; genetic disorders; infertility; male infertility; sperm morphology.
© The Author(s) 2025. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
None to declare.
Figures




References
-
- Adams A, Sriram A, Wayne Vogl A. Internalization of intact intercellular junctions in the testis by clathrin/actin-mediated endocytic structures: tubulobulbar complexes. Anat Rec (Hoboken) 2018;301:2080–2085. - PubMed
-
- Adham IM, Nayernia K, Burkhardt-Gottges E, Topaloglu O, Dixkens C, Holstein AF, Engel W. Teratozoospermia in mice lacking the transition protein 2 (Tnp2). Mol Hum Reprod 2001;7:513–520. - PubMed
-
- Aitken RJ, Paterson M, Fisher H, Buckingham DW, van Duin M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci 1995;108(Pt 5):2017–2025. - PubMed
-
- Albrecht M. Insights into the nature of human testicular peritubular cells. Ann Anat 2009;191:532–540. - PubMed
-
- Aldridge AC, Benson LP, Siegenthaler MM, Whigham BT, Stowers RS, Hales KG. Roles for Drp1, a dynamin-related protein, and milton, a kinesin-associated protein, in mitochondrial segregation, unfurling and elongation during Drosophila spermatogenesis. Fly (Austin) 2007;1:38–46. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

