Tumour extracellular vesicle surface Protein-mRNA integration assay for early detection of epithelial ovarian cancer
- PMID: 40803203
- PMCID: PMC12359246
- DOI: 10.1016/j.ebiom.2025.105884
Tumour extracellular vesicle surface Protein-mRNA integration assay for early detection of epithelial ovarian cancer
Abstract
Background: Early detection of epithelial ovarian cancer (EOC) is crucial for improving clinical outcomes. However, the sensitivity of primary serological marker cancer antigen 125 (CA125) is suboptimal for detecting early-stage EOC. Tumour-derived extracellular vesicles (EVs) are promising biomarkers for early cancer detection.
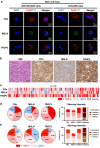
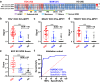
Methods: We developed an EOC EV Surface Protein-mRNA Integration (SPRI) Assay for early detection of EOC. This assay quantifies reference mRNAs within subpopulations of EOC EVs enriched by EV Click Beads targeting three EOC EV surface protein markers. Three EOC EV surface protein markers (i.e., FRα, MSLN, and TROP2) were selected through a bioinformatic framework using multi-omics data and underwent rigorous validation using EOC cell lines and EOC tissue microarrays. We then explored the translational potential of the EOC EV SPRI Assay through a phase II case-control study. The EOC EV SPRI Score was established using a logistic regression model in a training cohort (n = 118) and then validated in an independent validation cohort (n = 118).
Findings: EOC EV SPRI Score demonstrated superior performance for distinguishing EOC from benign ovarian masses and healthy donors with an area under the receiver operating characteristic (AUROC) of 0.99 (95% CI: 0.97-1.00) in the training cohort and 0.93 (95% CI: 0.88-0.97) in the validation cohort. It outperformed matched serum CA125, and the performance remained excellent in earlier stages of EOC (Stage I/II, AUROC = 0.93, 95% CI: 0.88-0.98) and the subgroup of high-grade serous carcinoma (AUROC = 0.97, 95% CI: 0.87-0.97).
Interpretation: The EOC EV SPRI assay demonstrated significant potential for early detection of EOC and improving long-term patient outcomes.
Funding: This work is supported by National Institutes of Health (R01CA277530, R01CA255727, R01CA253651, R01CA253651-04S1, R21CA280444, R01CA246304, U01EB026421, R44CA288163, U01CA271887, and U01CA230705), DOD (HT9425-23-1-0361) and OCRA (CRDG-2023-3-1000) for the U.S.
Study: Additionally, we acknowledge the support of the Science and Technology Foundation of Suzhou (SZS2023006, SSD2023004) and the Youth Innovation Promotion Association CAS (2023335) for the work conducted at SINANO.
Keywords: Biomarker; Epithelial ovarian cancer; Extracellular vesicle; Liquid biopsy.
Copyright © 2025. Published by Elsevier B.V.
Conflict of interest statement
Declaration of interests HRT is a co-founder and shareholder in Cytolumina Technologies Corp, Pulsar Therapeutics, and Eximius Diagnostics Corp. Y Zhu is a co-founder and shareholder in Eximius Diagnostics Corp.
Figures




References
-
- Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. - PubMed
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Lheureux S., Gourley C., Vergote I., Oza A.M. Epithelial ovarian cancer. Lancet. 2019;393(10177):1240–1253. - PubMed
-
- Lheureux S., Braunstein M., Oza A.M. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69(4):280–304. - PubMed
-
- Kuroki L., Guntupalli S.R. Treatment of epithelial ovarian cancer. BMJ. 2020;371 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

