A Cell Marker Atlas to Distinguish Metaplastic Transitions in Human Esophagus and Stomach
- PMID: 40803502
- PMCID: PMC12513315
- DOI: 10.1016/j.jcmgh.2025.101611
A Cell Marker Atlas to Distinguish Metaplastic Transitions in Human Esophagus and Stomach
Abstract
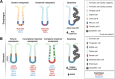
Metaplasia is an adaptative response to injury and inflammation and can be a precursor to dysplasia and cancer. Metaplasia in the esophagus, termed Barrett's esophagus, is the replacement of the stratified squamous epithelium by glandular tissue comprising gastric and/or intestinal cell lineages. Metaplasia in the stomach can be divided further into pyloric metaplasia, in which corpus glands become more antral-like, or gastric intestinal metaplasia (GIM), in which gastric cells are replaced by intestinal cell lineages, with the latter subdivided into complete and incomplete. The routine diagnosis of metaplasia and dysplasia is performed by examining hematoxylin and eosin-stained sections and mucin immunohistochemistry. However, these methods fail to capture the cellular diversity across glands and the molecular changes in cells that can predict possible progression to dysplasia or cancer. The use of immunohistochemistry- or immunofluorescence-based biomarkers can improve our understanding of gland phenotypes and aid the differentiation of metaplastic and dysplastic transitions. Here, we provide an overview of the pathophysiology of metaplasia in the esophagus and stomach and detail the current understanding of biomarker expression across metaplastic transitions. We suggest a cohort of biomarkers that can differentiate between metaplastic phenotypes in the esophagus (gastric-type and intestinal-type) and the stomach (pyloric metaplasia, incomplete GIM, and complete GIM) that might be used in research and clinical settings. Importantly, we detail the status of dysplasia biomarkers in both the esophagus and stomach, which may have clinical relevance in stratification of high-risk patients.
Keywords: Cell State; Dysplasia; Esophagus; Intestinal; Metaplasia; Pyloric; Stomach.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Paull A., Trier J.S., Dalton M.D., et al. The histologic spectrum of Barrett’s esophagus. N Engl J Med. 1976;295:476–480. - PubMed
-
- Rajendra S. Diagnosis and management of Barrett’s esophagus: an updated ACG Guideline. Am J Gastroenterol. 2022;117:1880. - PubMed
-
- Fitzgerald R.C., di Pietro M., Ragunath K., et al. British Society of Gastroenterology British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7–42. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

