Immunogenicity of adjuvanted recombinant zoster vaccine in patients with rheumatoid arthritis treated with upadacitinib: 60-week results from a randomised controlled trial substudy
- PMID: 40803820
- PMCID: PMC12352179
- DOI: 10.1136/rmdopen-2025-005521
Immunogenicity of adjuvanted recombinant zoster vaccine in patients with rheumatoid arthritis treated with upadacitinib: 60-week results from a randomised controlled trial substudy
Abstract
Objective: To evaluate the immunogenicity of adjuvanted recombinant zoster vaccine (RZV) in patients with rheumatoid arthritis receiving upadacitinib 15 mg once daily (QD) with background methotrexate.
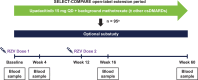
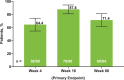
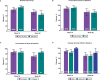
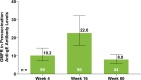
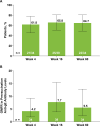
Methods: Eligible patients in SELECT-COMPARE (NCT02629159) receiving upadacitinib 15 mg QD and background methotrexate received RZV at weeks 0 and 12. Antibody titres were collected at weeks 0, 4, 16 and 60 (prevaccination, 4 weeks after first dose, and 4 and 48 weeks after second dose). The primary endpoint was the proportion of patients achieving a satisfactory humoral response to RZV at week 16 (≥ 4-fold increase in prevaccination anti-glycoprotein E (gE) antibody titres). Cell-mediated immune (CMI) response to RZV (≥ 2-fold increase in prevaccination gE-specific CD4+ [2+] T-cell frequency) was assessed at each time point in a subcohort of 38 patients. Safety was assessed for 30 days after each vaccination.
Results: Overall, 93 patients received both RZV doses (78.5% female; mean age, 62.4 years). At baseline, 49.5% used concomitant corticosteroids (median daily dose, 5.0 mg). Satisfactory humoral responses to RZV were observed in 87.8% (95% CI 81.0 to 94.5) of patients at week 16. Age and concomitant corticosteroid use did not affect RZV antibody response. Over 60% of patients achieved a CMI response to RZV at all time points. No serious adverse events were reported. One patient developed herpes zoster 4 months after the second RZV dose.
Conclusions: Most patients receiving upadacitinib 15 mg QD with background methotrexate achieved satisfactory humoral and CMI responses 4 weeks after the second RZV vaccination (week 16).
Keywords: Antirheumatic Agents; Arthritis, Rheumatoid; Vaccination.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: KLW has received consulting fees and/or research grants from AbbVie, Bristol Myers Squibb, Galapagos, Gilead, Lilly, Pfizer, Roche and UCB. CG has received medical fees to serve as principal investigator in AbbVie clinical trials. EM has received or has pending grants from Bristol Myers Squibb, Novartis, Pfizer and Roche. He has received honorarium from AbbVie, Amgen, AstraZeneca, GSK, Lilly, Pfizer, Roche, Sandoz and Sanofi; has provided writing assistance, medicines, equipment or administrative support to AbbVie, Pfizer and Roche; and has received payment for lectures including service on speakers bureaus from AbbVie, Alpine Immunology, Amgen, AZ, GSK, Hi Bio, Janssen, Lilly, Pfizer, Roche, Sandoz and Sanofi. AW has received honoraria from AbbVie, Amgen, Bristol Myers Squibb, Lilly, Novartis, Pfizer and Sanofi. ALC has received honoraria paid to his institution from AbbVie, BioCSL/Seqirus, GSK and Moderna. JK, SKP, YL, XB, IF and MC are full-time employees of AbbVie and may hold AbbVie stock or stock options.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
