Dopamine signaling drives skin invasion by human-infective nematodes
- PMID: 40804046
- PMCID: PMC12350745
- DOI: 10.1038/s41467-025-62517-z
Dopamine signaling drives skin invasion by human-infective nematodes
Abstract
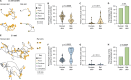
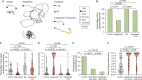
Skin-penetrating nematodes are one of the most prevalent causes of disease worldwide. The World Health Organization has targeted these parasites for elimination by 2030, but the lack of preventative measures is a major obstacle to this goal. Infective larvae enter hosts through skin and blocking skin penetration could prevent infection. However, in order to prevent worm ingress via the skin, an understanding of the behavioral and neural mechanisms that drive skin penetration is required. Here, we describe the skin-penetration behavior of the human-infective threadworm Strongyloides stercoralis. We show that S. stercoralis engages in repeated cycles of pushing, puncturing, and crawling on the skin surface before penetrating. Pharmacological inhibition of dopamine signaling inhibits these behaviors in S. stercoralis and the human hookworm Ancylostoma ceylanicum, suggesting a critical role for dopamine signaling in driving skin penetration across distantly related nematodes. CRISPR-mediated disruption of dopamine biosynthesis and chemogenetic silencing of dopaminergic neurons also inhibit skin penetration. Finally, inactivation of the TRPN channel TRP-4, which is expressed in the dopaminergic neurons, blocks skin penetration. Our results suggest that drugs targeting TRP-4 and other nematode-specific components of the dopaminergic pathway could be developed into topical prophylactics that block skin penetration, thereby preventing infections.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures







Update of
-
Dopamine signaling drives skin invasion by human-infective nematodes.bioRxiv [Preprint]. 2025 Jan 30:2025.01.29.635547. doi: 10.1101/2025.01.29.635547. bioRxiv. 2025. Update in: Nat Commun. 2025 Aug 13;16(1):7246. doi: 10.1038/s41467-025-62517-z. PMID: 39974984 Free PMC article. Updated. Preprint.
References
-
- Riaz, M. et al. Prevalence, risk factors, challenges, and the currently available diagnostic tools for the determination of helminths infections in human. Eur. J. Inflamm.18, 1–15 (2020).
MeSH terms
Substances
Grants and funding
- R01 AI175183/AI/NIAID NIH HHS/United States
- T32 GM145388/GM/NIGMS NIH HHS/United States
- T32 AI007323/AI/NIAID NIH HHS/United States
- DP2 AI184544/AI/NIAID NIH HHS/United States
- R01AR081337/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- T34 GM008563/GM/NIGMS NIH HHS/United States
- T32AI007323/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- F32AI174816/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01AI175183/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- T32GM145388/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- T34GM008563/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- F32 AI174816/AI/NIAID NIH HHS/United States
- DP2AI184544/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 AR081337/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

