An in vitro study exploring the role of mucin in the protein-flavor binding mechanism
- PMID: 40804055
- PMCID: PMC12350710
- DOI: 10.1038/s41538-025-00451-6
An in vitro study exploring the role of mucin in the protein-flavor binding mechanism
Abstract
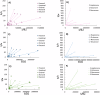
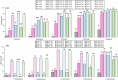
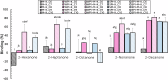
In the mouth, food flavors interact with salivary proteins like mucin, which protects mucosal surfaces and influences the rate of aroma release. The role of mucin in the protein-flavor binding mechanism was evaluated in a model system in vitro using GC-MS. The number of binding sites (n) and binding constants (K) of commercial food protein isolates (PIs) and carbonyl compounds were calculated using Klotz plots. Results suggested a linear relationship between PIs and carbonyl compounds, where K increased with the flavor chain length, and n ranged from n = 0.021 to 7.194. Mucin addition to flavor-protein systems increased flavor binding up to fifteen times. At 0.01(w/v)% mucin, structural changes enhanced flavor binding. At higher mucin levels, further unfolding leads to aggregation, restricting access of the flavor molecules to the binding sites. These results confirmed the role of flavor structural characteristics and mucin on flavor binding, essential for optimal food design.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: H.-G.J. is employed by Unilever, a multinational consumer goods company. The other authors declare no competing interests.
Figures

Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
[Analysis of volatile aroma components in tobacco by gas chromatography-mass spectrometry coupled with headspace solid phase microextraction].Se Pu. 2025 Jul;43(7):793-804. doi: 10.3724/SP.J.1123.2024.10004. Se Pu. 2025. PMID: 40610773 Free PMC article. Chinese.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
References
-
- Pagès-Hélary, S., Andriot, I., Guichard, E. & Canon, F. Retention effect of human saliva on aroma release and respective contribution of salivary mucin and α-amylase. Food Res. Int.64, 424–431 (2014). - PubMed
-
- Anantharamkrishnan, V., Hoye, T. & Reineccius, G. A. Covalent adduct formation between flavor compounds of various functional group classes and the model protein β-lactoglobulin. J. Agric. Food Chem.68, 6395–6402 (2020). - PubMed
-
- Bi, S. et al. Non-covalent interactions of selected flavors with pea protein: Role of molecular structure of flavor compounds. Food Chem.389, 133044 (2022). - PubMed
-
- Li, Y. et al. Spectroscopy combined with spatiotemporal multiscale strategy to study the adsorption mechanism of soybean protein isolate with meat flavor compounds (furan): Differences in position and quantity of the methyl. Food Chem.451, 139415 (2024). - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

