Mechanodynamic brain on chip for studying human stem cell derived neuronal networks
- PMID: 40804068
- PMCID: PMC12350671
- DOI: 10.1038/s41598-025-14187-6
Mechanodynamic brain on chip for studying human stem cell derived neuronal networks
Abstract
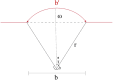
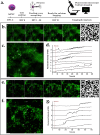
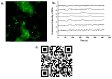
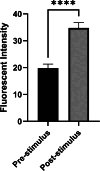
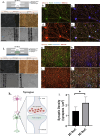
Brain tissue orchestrates neuronal function through biochemical and mechanical cues. Utilizing in vitro modeling, often the dynamics of mechanical aspects in neuronal cell cultures is neglected. However, the growing recognition of the importance of mechanical cues in neural development and healthy brain function necessitates a shift in how we study cultured neurons. Microfluidic platforms, like a Brain-on-Chip (BoC), can take active mechanical stimuli into account. In our BoC design a set of microchannels manufactured in a glass substrate by FEMTOprint technology is assembled with a spin-coated polydimethylsiloxane (PDMS) membrane and a PDMS culture chamber, which was fabricated from a stereolithographically made mold by replication. The membrane can locally deform across the culture chamber by air pressure. This paper describes the design, fabrication and test of such a novel BoC, offering an experimental setting in which we demonstrated mechano-dynamic elevated Calcium signaling in cultured human induced neural stem cell-derived neuronal networks.
Keywords: Brain-on-Chip (BoC); Calcium live imaging; HiPSCs-derived neurons; Mechanical stimulation; Neuronal activity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures









Similar articles
-
Crafting Precision: Design and Fabrication of a Xurography-Driven Microfluidic Platform for Exploring Neuron Culture and Targeted Drug Screening.ACS Chem Neurosci. 2025 Aug 6;16(15):2785-2799. doi: 10.1021/acschemneuro.5c00016. Epub 2025 Jul 11. ACS Chem Neurosci. 2025. PMID: 40641433
-
Novel application of metabolic imaging of early embryos using a light-sheet on-a-chip device: a proof-of-concept study.Hum Reprod. 2025 Jan 1;40(1):41-55. doi: 10.1093/humrep/deae249. Hum Reprod. 2025. PMID: 39521726 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Three-dimensional human neural culture on a chip recapitulating neuroinflammation and neurodegeneration.Nat Protoc. 2023 Sep;18(9):2838-2867. doi: 10.1038/s41596-023-00861-4. Epub 2023 Aug 4. Nat Protoc. 2023. PMID: 37542184 Review.
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
References
-
- Guo, T., Ren, P., Hao, S. & Wang, B. The underestimated role of mechanical stimuli in brain diseases and the related in vitro models. Curr. Pharmaceutical Design. 23 (2016). - PubMed
-
- Chicurel, M. Cell migration research is on the move. Science295, 606–609 (2002). - PubMed
-
- Tyler, W. J. The mechanobiology of brain function. Nat. Rev. Neurosci.13, 867–878 (2012). - PubMed
-
- Cipolla, M. J. Control of cerebral blood flow. in The Cerebral Circulation. (Morgan & Claypool Life Sciences, 2009). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

