rTMS ameliorates cerebral ischemia-reperfusion injury by inhibiting Golgi apparatus stress through epigenetic modulation of Gli2
- PMID: 40804340
- PMCID: PMC12350837
- DOI: 10.1038/s42003-025-08613-8
rTMS ameliorates cerebral ischemia-reperfusion injury by inhibiting Golgi apparatus stress through epigenetic modulation of Gli2
Abstract
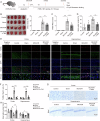
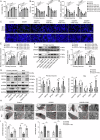
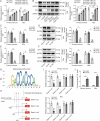
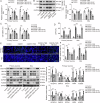
Cerebral ischemic stroke represents a primary cause of permanent disability and mortality globally. Repetitive transcranial magnetic stimulation (rTMS) has emerged as a prominent focus in treating a wide range of neurological disorders. In this study, we explore the role of rTMS in alleviating cerebral ischemia-reperfusion (I/R) injury by mediating Golgi apparatus (GA) stress. Here, we find that rTMS upregulates Dram1 expression and ameliorates GA stress in cerebral I/R injury in vivo and in vitro. Gli2 transcriptionally activates Dram1. HDAC5 inhibits H3K27ac modification of Gli2 promoter. rTMS promotes Gli2 expression by inhibiting HDAC5. Gli2 knockdown reverses the inhibitory effect of rTMS on OGD/R-induced neuronal GA stress. In conclusion, rTMS inhibits HDAC5-mediated deacetylation of Gli2 promoter to promote the transcriptional activation of Dram1, thereby suppressing cerebral I/R-induced GA stress. Targeting Gli2/ Dram1 axis may be an effective way to enhance the anti-ischemic stroke effect of rTMS.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Ginsenoside Rg1 mitigates cerebral ischaemia/reperfusion injury in mice by inhibiting autophagy through activation of mTOR signalling.Acta Pharmacol Sin. 2024 Dec;45(12):2474-2486. doi: 10.1038/s41401-024-01334-4. Epub 2024 Jun 27. Acta Pharmacol Sin. 2024. PMID: 38937576
-
Remote Ischemic Postconditioning Improve Cerebral Ischemia-Reperfusion Injury Induced Cognitive Dysfunction through Suppressing Mitochondrial Apoptosis in Hippocampus via TK/BK/B2R-Mediated PI3K/AKT.Mol Neurobiol. 2025 Aug;62(8):10652-10669. doi: 10.1007/s12035-025-04864-y. Epub 2025 Apr 14. Mol Neurobiol. 2025. PMID: 40229456 Free PMC article.
-
Repetitive transcranial magnetic stimulation for post-traumatic stress disorder in adults.Cochrane Database Syst Rev. 2024 Aug 2;8(8):CD015040. doi: 10.1002/14651858.CD015040.pub2. Cochrane Database Syst Rev. 2024. PMID: 39092744 Free PMC article.
-
Transcranial direct current stimulation enhances the protective effect of isoflurane preconditioning on cerebral ischemia/reperfusion injury: A new mechanism associated with the nuclear protein Akirin2.CNS Neurosci Ther. 2024 Sep;30(9):e70033. doi: 10.1111/cns.70033. CNS Neurosci Ther. 2024. PMID: 39267282 Free PMC article.
-
Non-invasive brain stimulation techniques for chronic pain.Cochrane Database Syst Rev. 2018 Mar 16;3(3):CD008208. doi: 10.1002/14651858.CD008208.pub4. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2018 Apr 13;4:CD008208. doi: 10.1002/14651858.CD008208.pub5. PMID: 29547226 Free PMC article. Updated.
References
-
- Mao, Z. et al. Ligustilide ameliorates hippocampal neuronal injury after cerebral ischemia reperfusion through activating PINK1/Parkin-dependent mitophagy. Phytomedicine101, 154111 (2022). - PubMed
-
- Lan, Z. et al. Curcumin-primed olfactory mucosa-derived mesenchymal stem cells mitigate cerebral ischemia/reperfusion injury-induced neuronal PANoptosis by modulating microglial polarization. Phytomedicine129, 155635 (2024). - PubMed
-
- Xu, D. et al. Orexin-A alleviates astrocytic apoptosis and inflammation via inhibiting OX1R-mediated NF-κB and MAPK signaling pathways in cerebral ischemia/reperfusion injury. Biochim Biophys. Acta Mol. Basis Dis.1867, 166230 (2021). - PubMed
LinkOut - more resources
Full Text Sources

