Malaria-malnutrition interaction: prevalence, risk factors, and the impact of intermittent preventive therapy for malaria on nutritional status of school-age children in Muheza, Tanga, Tanzania - A cross-sectional survey and a randomized controlled open-label trial
- PMID: 40804388
- PMCID: PMC12351946
- DOI: 10.1186/s12889-025-23315-w
Malaria-malnutrition interaction: prevalence, risk factors, and the impact of intermittent preventive therapy for malaria on nutritional status of school-age children in Muheza, Tanga, Tanzania - A cross-sectional survey and a randomized controlled open-label trial
Abstract
Background: WHO and the Lancet reported that malaria and malnutrition form a double health burden in low and middle-income countries. Despite the massive implementation of malaria control interventions, there is scarce evidence on the impact of intermittent preventive therapy (IPTsc) for malaria on the nutritional status of school-age children. In this study, we aimed to determine malnutrition risk factors and evaluate the impact of IPTsc for malaria on the nutritional status of school-age children in Muheza, Tanga, Tanzania.
Methods: We analysed secondary data from a cross-sectional baseline survey and a randomized controlled open-label trial conducted in Muheza, Tanga, Tanzania. Participants of our study were children of age 5-15 years. Baseline data collection was carried out between February-April 2019. The study continued through December 2020, during which participants were randomly assigned to one of the three groups: dihydroartemisinin-piperaquine (DP), artesunate-amodiaquine (ASAQ), or a control group, using a 3-arms balanced block design with a 1:1:1 allocation ratio. Intervention treatments were administered at recruitment, 4 months, and 8 months of the trial. Data were analysed using logistic regression and a linear mixed model.
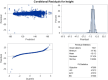
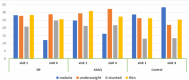
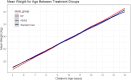
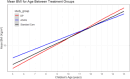
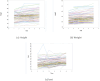
Findings: At baseline, the prevalence of malaria was 27%. The prevalence of being underweight among children of ≤ 10 years was 23%. Among all children surveyed at baseline, 21% were stunted and 28% were either thin or severely thin. The odds of stunting were 78% higher (AOR = 1.78, 95%CI = [1.36, 2.33], P < 0.001) among children who had malaria compared with those who did not have malaria. Children from low socioeconomic status (SES) had higher odds of being underweight (AOR = 1.50, 95%CI = [1.13,2.01], P = 0.006) compared with their high SES counterparts. During the intervention, change in mean weight, height, and BMI over time as estimated from age-treatment interaction was not significantly different in the dihydroartemisinin-piperaquine (DP) and Artesunate amodiaquine (ASAQ) treatment groups compared with the control group.
Conclusion: Although substantial efforts to control malaria are ongoing in the study setting, the dual burden of malaria and malnutrition remains significant. Anti-malaria use for preventive purpose may not be sufficient to improve nutritional status, reinforcing that integrated interventions are required to address both malaria and malnutrition. Public health efforts should combine malaria control with nutrition programs, including community-driven strategies to enhance sustainable nutrition education and access to adequate food at home and school. Protocol for the parent study that generated these data was registered with ClinicalTrials.gov (NCT03640403) on Aug 21, 2018.
Keywords: Intermittent preventive therapy; Malaria; Malnutrition; Nutrition in children and adolescents.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This manuscript is based on secondary data analysis from a cross-sectional survey and a clinical trial for which ethical clearance was obtained during the primary data collection. This study adhered to the principles outlined in the Declaration of Helsinki concerning ethical research involving human subjects. Prior to participation in the original study, both children and their parents were thoroughly informed about the trial's objectives and procedures. Parents or guardians of eligible children provided their consent for participation, while children aged 11 years and older were given the opportunity to assent to participate in the study. The study acquired regulatory approval from the Medical Research Coordination Committee (MRCC) of the National Institute of Medical Research (NIMR), Tanzania. The initial approval based on the protocol was granted on 20 th July 2018. The study protocol was amended, reviewed by the committee, and approved on 19 th March 2019 with validity date on 19 th July 2019. However, based on the progress report of July 2019, the ethical clearance committee granted study extension until 20 th July 2020. The study approval numbers were NIMR/HQ/R.8a/Vol.IX/2818 and NIMR/HQ/R.8c/Vol.I/668 (for amendment) also NIMR/HQ/R.8c/Vol.I/1276 for ethical clearance extension. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- World Health Organization. World malaria report 2017. Geneva: World Health Organization; 2017. 158 p. Available from: https://iris.who.int/handle/10665/259492. Cited 2025 Mar 25
-
- World malaria report 2024: addressing inequity in the global malaria response. Geneva: World Health Organization; 2024. Licence: CC BY-NC-SA 3.0 IGO.
-
- World malaria report 2020: 20 years of global progress and challenges. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO.
-
- Organization WH. World Malaria Report 2021. Geneva: World Health Organization; 2021.
-
- Nankabirwa JI, Wandera B, Amuge P, Kiwanuka N, Dorsey G, Rosenthal PJ, et al. Impact of intermittent preventive treatment with dihydroartemisinin-piperaquine on malaria in Ugandan schoolchildren: a randomized, placebo-controlled trial. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014May;58(10):1404–12. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

